Catalytic Mechanism of SARS-CoV-2 3-Chymotrypsin-Like Protease as Determined by Steady-State and Pre-Steady-State Kinetics
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
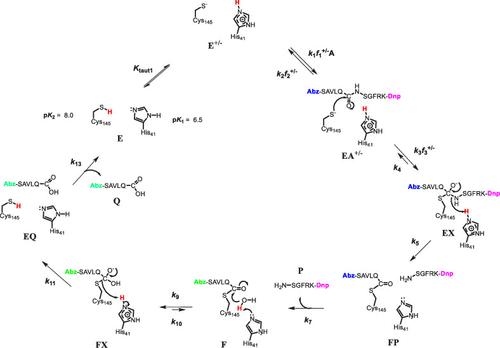
The 3-chymotrypsin-like protease (3CL-PR; also known as Main protease) of SARS-CoV-2 is a cysteine protease that is the target of the COVID-19 drug, Paxlovid. Here, we report for 3CL-PR, the pH-rate profiles of a substrate, an inhibitor, affinity agents, and solvent kinetic isotope effects (sKIEs) obtained under both steady-state and pre-steady-state conditions. “Bell-shaped” plots of log(kcat/Ka) vs pH for the substrate (Abz)SAVLQ*SGFRK(Dnp)-NH2 and pKi vs pH for a peptide aldehyde inhibitor demonstrated that essential acidic and basic groups of pK2 = 8.2 ± 0.4 and pK1 = 6.2 ± 0.3, respectively, are required for catalysis, and the pH-dependence of inactivation of 3CL-PR by iodoacetamide and diethylpyrocarbonate identified enzymatic groups of pK2 = 7.8 ± 0.1 and pK1 = 6.05 ± 0.07, which must be unprotonated for maximal inactivation. These data are most consistent with the presence of a neutral catalytic dyad (Cys-SH-His) in the 3CL-PR free enzyme, with respective pK values for the cysteine and histidine groups of pK2 = 8.0 and pK1 = 6.5. The steady-state sKIEs were D2O(kcat/Ka) = 0.56 ± 0.05 and D2Okcat = 1.0 ± 0.1, and sKIEs indicated that the Cys-S–-HisH+ tautomer was enriched in D2O. Presteady-state kinetic analysis of (Abz)SAVLQ*SGFRK(Dnp)-NH2 exhibited transient lags preceding steady-state rates, which were considerably faster in D2O than in H2O. The transient rates encompass steps that include substrate binding and acylation, and are faster in D2O wherein the more active Cys-S–-HisH+ tautomer predominates. A full catalytic mechanism for 3CL-PR is proposed.
稳态和前稳态动力学确定的 SARS-CoV-2 3-Chymotrypsin-Like 蛋白酶催化机理
SARS-CoV-2 的 3-糜蛋白酶样蛋白酶(3CL-PR;又称主蛋白酶)是一种半胱氨酸蛋白酶,是 COVID-19 药物 Paxlovid 的靶标。在此,我们报告了 3CL-PR 在稳态和预稳态条件下底物、抑制剂、亲和剂和溶剂动力学同位素效应(sKIEs)的 pH-速率曲线。底物 (Abz)SAVLQ*SGFRK(Dnp)-NH2 的 log(kcat/Ka) vs pH 和肽醛抑制剂的 pKi vs pH 的 "钟形 "图表明,pK2 = 8.2 ± 0.4 和 pK1 = 6.2 ± 0.碘乙酰胺和碳酸二乙酯使 3CL-PR 失活的 pH 值依赖性确定了 pK2 = 7.8 ± 0.1 和 pK1 = 6.05 ± 0.07 的酶基,这些酶基必须是非质子化的,才能最大程度地失活。这些数据最符合 3CL-PR 游离酶中存在中性催化二元(Cys-SH-His)的情况,半胱氨酸和组氨酸基团的 pK 值分别为 pK2 = 8.0 和 pK1 = 6.5。稳态 sKIEs 分别为 D2O(kcat/Ka) = 0.56 ± 0.05 和 D2Okcat = 1.0 ± 0.1,sKIEs 表明 Cys-S--HisH+ 同系物在 D2O 中富集。(Abz)SAVLQ*SGFRK(Dnp)-NH2的预稳态动力学分析表明,稳态速率之前存在瞬时滞后,在D2O中比在H2O中快很多。瞬时速率包括底物结合和酰化等步骤,在D2O中更快,因为在D2O中活性更高的Cys-S--HisH+同系物占主导地位。提出了 3CL-PR 的完整催化机理。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


