Synovial tissue myeloid dendritic cell subsets exhibit distinct tissue-niche localization and function in health and rheumatoid arthritis
IF 25.5
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
Current rheumatoid arthritis (RA) treatments do not restore immune tolerance. Investigating dendritic cell (DC) populations in human synovial tissue (ST) may reveal pathways to reinstate tolerance in RA. Using single-cell and spatial transcriptomics of ST biopsies, as well as co-culture systems, we identified condition- and niche-specific DC clusters with distinct functions. Healthy tissue contained tolerogenic AXL+ DC2s in the lining niche. In active RA, the hyperplasic lining niche was populated with inflammatory DC3s that activated CCL5-positive effector memory T cells, promoting synovitis. Lymphoid niches that emerged in the sublining layer were enriched with CCR7+ DC2s, which interacted with naive T cells, potentially driving the local expansion of new effector T cells. Remission saw the resolution of these pathogenic niches but lacked recovery of tolerogenic DC2s and exhibited activation of blood precursors of ST-DC3 clusters prior to flare-ups. Targeting pathogenic DC3s or restoring tolerogenic DC2s may help restore immune homeostasis in RA joints.
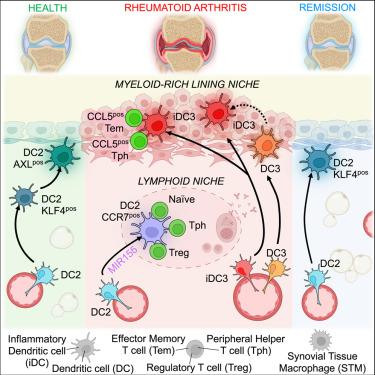
滑膜组织髓系树突状细胞亚群在健康和类风湿性关节炎中表现出不同的组织特异性定位和功能
目前的类风湿性关节炎(RA)治疗无法恢复免疫耐受。研究人体滑膜组织(ST)中的树突状细胞(DC)群可能会揭示恢复类风湿性关节炎耐受性的途径。利用ST活检组织的单细胞和空间转录组学以及共培养系统,我们发现了具有不同功能的条件和生态位特异性DC群。健康组织的内衬龛中含有耐受性AXL+ DC2。在活动性RA中,增生的内衬龛中充满了炎性DC3,它们能激活CCL5阳性的效应记忆T细胞,促进滑膜炎的发生。在衬里下层出现的淋巴龛富含CCR7+ DC2s,它们与幼稚T细胞相互作用,可能推动新的效应T细胞在局部扩增。在缓解期,这些致病性龛位得到了解决,但缺乏耐受性DC2的恢复,并且在复发前ST-DC3集群的血液前体被激活。针对致病性DC3或恢复耐受性DC2可能有助于恢复RA关节的免疫平衡。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Immunity
医学-免疫学
CiteScore
49.40
自引率
2.20%
发文量
205
审稿时长
6 months
期刊介绍:
Immunity is a publication that focuses on publishing significant advancements in research related to immunology. We encourage the submission of studies that offer groundbreaking immunological discoveries, whether at the molecular, cellular, or whole organism level. Topics of interest encompass a wide range, such as cancer, infectious diseases, neuroimmunology, autoimmune diseases, allergies, mucosal immunity, metabolic diseases, and homeostasis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


