The vertebrate segmentation clock drives segmentation by stabilizing Dusp phosphatases in zebrafish
IF 10.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
Pulsatile activity of the extracellular signal-regulated kinase (ERK) controls several cellular, developmental, and regenerative programs. Sequential segmentation of somites along the vertebrate body axis, a key developmental program, is also controlled by ERK activity oscillation. The oscillatory expression of Her/Hes family transcription factors constitutes the segmentation clock, setting the period of segmentation. Although oscillation of ERK activity depends on Her/Hes proteins, the underlying molecular mechanism remained mysterious. Here, we show that Her/Hes proteins physically interact with and stabilize dual-specificity phosphatases (Dusp) of ERK, resulting in oscillations of Dusp4 and Dusp6 proteins. Pharmaceutical and genetic inhibition of Dusp activity disrupt ERK activity oscillation and somite segmentation in zebrafish. Our results demonstrate that post-translational interactions of Her/Hes transcription factors with Dusp phosphatases establish the fundamental vertebrate body plan. We anticipate that future studies will identify currently unnoticed post-translational control of ERK pulses in other systems.
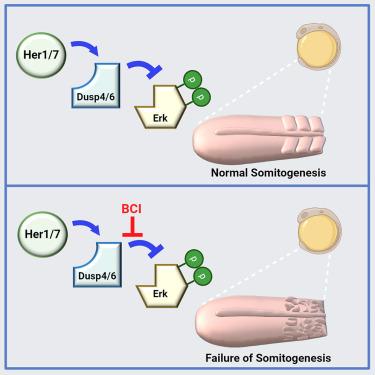
脊椎动物分割钟通过稳定斑马鱼体内的 Dusp 磷酸酶来驱动分割
细胞外信号调节激酶(ERK)的脉冲活动控制着多个细胞、发育和再生程序。脊椎动物体轴上体节的顺序分割是一种关键的发育程序,也受ERK活性振荡的控制。Her/Hes家族转录因子的振荡表达构成了分割时钟,设定了分割的周期。虽然ERK活性的振荡依赖于Her/Hes蛋白,但其分子机制仍然神秘莫测。在这里,我们发现 Her/Hes 蛋白与 ERK 的双特异性磷酸酶(Dusp)发生物理相互作用并使其稳定,从而导致 Dusp4 和 Dusp6 蛋白的振荡。药物和基因抑制 Dusp 的活性会破坏 ERK 活性振荡和斑马鱼体节的分割。我们的研究结果表明,Her/Hes 转录因子与 Dusp 磷酸化酶的翻译后相互作用建立了脊椎动物的基本身体计划。我们预计,未来的研究将在其他系统中发现目前尚未注意到的ERK脉冲翻译后控制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Developmental cell
生物-发育生物学
CiteScore
18.90
自引率
1.70%
发文量
203
审稿时长
3-6 weeks
期刊介绍:
Developmental Cell, established in 2001, is a comprehensive journal that explores a wide range of topics in cell and developmental biology. Our publication encompasses work across various disciplines within biology, with a particular emphasis on investigating the intersections between cell biology, developmental biology, and other related fields. Our primary objective is to present research conducted through a cell biological perspective, addressing the essential mechanisms governing cell function, cellular interactions, and responses to the environment. Moreover, we focus on understanding the collective behavior of cells, culminating in the formation of tissues, organs, and whole organisms, while also investigating the consequences of any malfunctions in these intricate processes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


