Aqueous two-phase systems for environmentally friendlier separation of rare earth elements and transition metals: Applications and new molecular insights
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
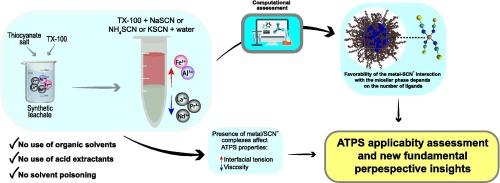
Aqueous two-phase systems (ATPSs) have been proposed as an alternative liquid–liquid extraction technique applicable for the separation of economically valuable metals. However, gaps in knowledge and the need for a deeper understanding of the mechanisms involved in metal extraction still hinder the widespread adoption of these systems. In this study, ATPSs formed by TX-100 and electrolytes were employed to separate La, Pr, and Nd from Al and Fe. Use of an ATPS composed of TX-100, NH4SCN, and water, at pH 2.00, with a phase ratio of 1:5, resulted in total extraction of Al and Fe, although there was also considerable co-extraction of REEs. When a phase ratio of 1:2 was used for an ATPS composed of TX-100, (NH4)2SO4, and water, with NH4SCN as extractant, at pH 1.00, it was possible to separate Fe from the other metals, with significantly lower co-extraction of REEs and Al (%ELa = 4.05 %, %EPr = 6.19 %, %ENd = 4.87 %, %EFe = 98.61 %, %EAl = 2.54 %). The results of density functional theory (DFT) calculations correlated well with the experimental metal extraction data, demonstrating that the characteristics and solvation of the complexes formed in the ATPS played a fundamental role in metal partitioning. Measurements of the physical properties of the ATPS phases revealed variations in viscosity and interfacial tension values, which could assist in developing applications on a larger scale. The findings demonstrated that ATPSs could be used to separate REEs from transition metals, with potential applications in the recovery of REEs present in secondary sources.
用于分离稀土元素和过渡金属的环保型水相两相系统:应用和新的分子认识
水溶液两相萃取系统(ATPS)已被提出作为一种替代性液液萃取技术,适用于分离具有经济价值的金属。然而,知识上的差距和对金属萃取机理的深入理解仍然阻碍着这些系统的广泛应用。本研究采用 TX-100 和电解质形成的 ATPS 从铝和铁中分离出 La、Pr 和 Nd。使用由 TX-100、NH4SCN 和水组成的 ATPS,pH 值为 2.00,相比为 1:5,可完全萃取出铝和铁,但也有相当多的 REEs 被共萃取。在pH值为1.00的条件下,对由TX-100、(NH4)2SO4和水组成的ATPS(以NH4SCN为萃取剂)采用1:2的相比时,可以将铁从其他金属中分离出来,同时显著降低了REEs和Al的共萃取率(%ELa = 4.05 %, %EPr = 6.19 %, %ENd = 4.87 %, %EFe = 98.61 %, %EAl = 2.54 %)。密度泛函理论(DFT)计算的结果与金属萃取实验数据密切相关,这表明 ATPS 中形成的络合物的特性和溶解度在金属分配中起着根本性的作用。对 ATPS 相的物理性质的测量显示了粘度和界面张力值的变化,这有助于开发更大规模的应用。研究结果表明,ATPS 可用于从过渡金属中分离 REEs,在回收二次资源中的 REEs 方面具有潜在的应用价值。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


