Valorization of pomegranate waste through green solvent extraction and biochar production: a zero-waste biorefinery approach†
IF 9.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
This study introduces a sustainable, zero-waste biorefinery approach for the valorization of pomegranate (Punica granatum) waste, focusing on the sequential extraction of anthocyanins, ellagic acid and its derivatives using environmentally friendly solvents, followed by biochar production. Initially, a COSMO-RS in silico analysis was conducted, screening 10 512 combinations of hydrogen bond acceptors (HBAs) and hydrogen bond donors (HBDs) typically used in eutectic solvent formulations, along with 49 bio-based solvents, to identify the most efficient green solvents for recovering anthocyanins, ellagic acid and its derivatives. In the first step, an aqueous solution of gamma-valerolactone (GVL) (2900 mM, pH 2) was used for solid–liquid extraction; this led to the optimization of extraction conditions (solid–liquid ratio of 0.07 gbiomass mLsolvent−1, at 25 °C for 55 minutes) yielding 38.52 ± 0.06 mganthocyanins gbiomass−1. Subsequently, the residual biomass underwent a second extraction using an aqueous solution of the ionic liquid (IL) cholinium acetate (2900 mM, pH 13) under similar conditions, yielding a rich fraction of ellagic acid and its derivatives (21.82 mgellagic acid gbiomass−1). The remaining biomass was then converted into activated biochar using a eutectic solvent composed of cholinium chloride and oxalic acid (molar ratio 1 HBA : 2 HBD), providing a greener alternative to traditional biochar production methods. The resulting biochar was utilized as an adsorbent for removing synthetic dyes (food and textile) from aqueous solutions, presenting new opportunities for the remediation of contaminated water effluents. This zero-waste process fully valorizes pomegranate residues, adhering to green extraction principles and achieving a Path2Green score of 0.401 (corresponding to around 288.50 gCO2 gbiomass−1), underscoring its eco-friendliness. By minimizing waste and reducing the need for harmful organic solvents, this biorefinery model highlights the potential for greener industrial practices through the use of bio-based solvents and the complete utilization of biomass.
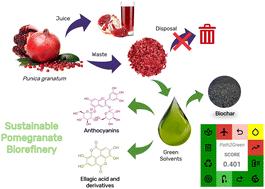
通过绿色溶剂萃取和生物炭生产实现石榴废弃物的价值化:一种零废弃物生物精炼方法†。
本研究介绍了一种用于石榴(Punica granatum)废弃物价值化的可持续、零废弃生物精炼方法,重点是使用环境友好型溶剂依次提取花青素、鞣花酸及其衍生物,然后生产生物炭。首先,进行了 COSMO-RS 硅分析,筛选了共晶溶剂配方中通常使用的 10 512 种氢键受体(HBA)和氢键供体(HBD)组合,以及 49 种生物基溶剂,以确定回收花青素、鞣花酸及其衍生物最有效的绿色溶剂。第一步,使用γ-戊内酯(GVL)水溶液(2900 mM,pH 2)进行固液萃取;优化了萃取条件(固液比为 0.07 gbiomass mLsolvent-1,25 °C,55 分钟),得到 38.52 ± 0.06 mganthocyanins gbiomass-1。随后,在类似条件下使用离子液体(IL)乙酸胆碱水溶液(2900 毫摩尔,pH 13)对残留生物质进行第二次萃取,得到富含鞣花酸及其衍生物的部分(21.82 毫克鞣花酸 gbiomass-1)。然后,利用氯化胆碱和草酸(摩尔比为 1 HBA : 2 HBD)组成的共晶溶剂将剩余的生物质转化为活性生物炭,为传统的生物炭生产方法提供了一种更环保的替代方法。生成的生物炭被用作一种吸附剂,用于去除水溶液中的合成染料(食品和纺织品),为受污染水体的修复提供了新的机遇。这种零废物工艺充分利用了石榴残渣的价值,坚持绿色提取原则,Path2Green 得分达到 0.401(相当于约 288.50 gCO2 gbiomass-1),突出了其生态友好性。通过最大限度地减少废物和降低对有害有机溶剂的需求,该生物精炼厂模式通过使用生物基溶剂和生物质的完全利用,凸显了绿色工业实践的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


