Rational design of the La-doped CuCoAl hydrotalcite catalyst for selective hydrogenation of furfuryl alcohol to 1,5-pentanediol†
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
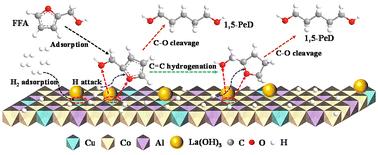
1,5-Pentanediol (1,5-PeD) is an important raw material for the preparation of degradable polyesters, polyurethanes and pharmaceutical intermediates. Efficient synthesis of 1,5-PeD from biomass-derived furfuryl alcohol (FFA) by hydrogenation is a green synthetic route instead of using fossil raw material production. Nevertheless, it suffers from great challenges as the various adsorption configurations of FFA on the catalyst surface induce diverse product distributions and low selectivity for 1,5-PeD. Herein, a CuCoAl hydrotalcite catalyst modified by La was fabricated and applied in the hydrogenation of FFA to 1,5-PeD. The results demonstrated that in the catalyst doped with La via deposition–precipitation methods (La/CuCoAl-DP) there appeared a strong Cu–La interaction, and it exhibited superior activity compared with other catalysts. A near 60% yield of 1,5-PeD was achieved under 160 °C, 4 MPa H2 within 2 h. Extensive characterizations including XRD, HRTEM, N2O-TPD and CO2-TPD demonstrated that the doping of La improved markedly the dispersion of Cu and the concentration of strong basic sites. Furthermore, HRTEM and the in situ XPS characterization verified that the addition of La species promoted the formation of a Cu–La interface with a stable Cun+–O–La(OH)3 structure on the catalyst surface. Such Cun+–O–La(OH)3 sites can simultaneously activate the furan ring and the –OH group in FFA with an intermediate six-membered ring transition state, leading to high selective cleavage of the C2–O1 bond in the furan ring to 1,5-PeD. Meanwhile, the DFT calculation results corroborated that the modifying by La species remarkably promoted the C2-end tilted adsorption of FFA on the catalyst surface and enhanced the ability of the catalyst to activate hydrogen. This study provided a new strategy for the high-value utilization of biomass resources and the development of multi-center catalysts.
用于糠醇选择性加氢制 1,5-戊二醇的掺镧铜铝氢铝酸盐催化剂的合理设计†
1,5-戊二醇(1,5-PeD)是制备可降解聚酯、聚氨酯和医药中间体的重要原料。通过氢化从生物质衍生的糠醇 (FFA) 中高效合成 1,5-PeD 是一条替代化石原料生产的绿色合成路线。然而,由于 FFA 在催化剂表面的吸附构型各异,导致产物分布不一,对 1,5-PeD 的选择性较低,因此该方法面临着巨大的挑战。本文制备了一种由 La 改性的铜钴铝氢铝酸盐催化剂,并将其应用于氢化反式脂肪酸制取 1,5-PeD 的过程。研究结果表明,通过沉积-沉淀法掺杂 La 的催化剂(La/CuCoAl-DP)具有很强的 Cu-La 相互作用,其活性优于其他催化剂。包括 XRD、HRTEM、N2O-TPD 和 CO2-TPD 在内的大量特性分析表明,掺入 La 能显著改善铜的分散性和强碱性位点的浓度。此外,HRTEM 和原位 XPS 表征验证了 La 物种的添加促进了催化剂表面形成具有稳定 Cun+-O-La(OH)3 结构的 Cu-La 界面。这种 Cun+-O-La(OH)3 位点可以同时激活呋喃环和 FFA 中的 -OH 基团,并具有中间六元环过渡态,从而导致呋喃环中的 C2-O1 键被高选择性地裂解为 1,5-PeD。同时,DFT 计算结果证实,La 物种的改性显著促进了催化剂表面对 FFA 的 C2 端倾斜吸附,增强了催化剂活化氢气的能力。该研究为生物质资源的高值化利用和多中心催化剂的开发提供了新的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


