Photo-driven reduction/cyclization of nitroarenes via electron donor–acceptor complexes: a novel method for the acquisition of N-heterocycles†
IF 9.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
A method based on an electron donor–acceptor (EDA) complex is presented for the one-step reduction/cyclization of nitroarenes to obtain N-heterocycles. This photo-mediated mode showcases the versatility of amines, which act as electron donors in assembling photosensitive species and serve as C1 synthons in C–N bond formation. In addition to its excellent tolerance towards various functional groups, this strategy exhibits remarkable applicability for the late-stage modification of drug molecules, delivering 26 examples of benzimidazoles and 29 examples of quinazolinones. Meanwhile, it displays a preferable EcoScale score and is considered acceptable in terms of economic viability and safety, aligning with the principles of green chemistry. Overall, this metal-free method offers controllable synthesis conditions, employs scalable flow technology with high space–time efficiency and demonstrates successful gram-scale application, thereby highlighting its significant potential for constructing bioactive N-heterocycles.
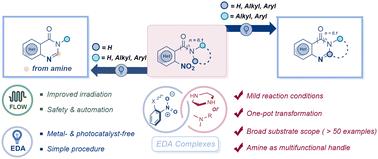
通过电子供体-受体复合物实现硝基烯烃的光驱动还原/环化:一种获得 N-杂环的新方法†。
本文介绍了一种基于电子供体-受体(EDA)复合物的方法,用于一步还原/环化硝基烯烃,从而获得 N-杂环。这种光介导模式展示了胺的多功能性,胺在组装光敏物种时充当电子供体,在形成 C-N 键时充当 C1 合子。除了对各种官能团具有极佳的耐受性外,这种策略在药物分子的后期修饰方面也具有显著的适用性,可提供 26 种苯并咪唑和 29 种喹唑啉酮。同时,它还显示出较好的生态尺度(EcoScale)得分,在经济可行性和安全性方面也被认为是可以接受的,符合绿色化学的原则。总之,这种无金属方法提供了可控的合成条件,采用了具有高时空效率的可扩展流动技术,并展示了克级规模的成功应用,从而凸显了其在构建具有生物活性的 N-杂环方面的巨大潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


