C–P Bond Cleavage Through Hydrogenation in Ruthenium Complexes Supported by P,N Ligands
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
The reactivity of ruthenium hydride complexes supported by 2-((di-tert-butylphosphaneyl)methyl)pyridine, L1 and 2-(di-tert-butylphosphaneyl)pyridine, L2, was explored. The reaction of {Ru(COD)Cl2}x with L1 in the presence of base and 10 bar of H2 gave the expected complex [Ru(L1)2(H)Cl], 1, while the same reaction with L2 gave [Ru(L2)(P(H)tBu2)(H)Cl], 2, that results from the cleavage of a C–P bond. We were able to establish that under the reaction conditions the first species formed is [Ru(L2)2(H)Cl], 3, and that this species decomposes to give complex 2 and is in equilibrium with [Ru(L2)2Cl2], 4. The proposed mechanism obtained by DFT has the protonation of the carbon as the highest energy step (38.9 kcal/mol), consistent with a slow reaction. Preliminary studies reveal that complex 2 is a very active catalyst in the hydrogenation of benzaldehyde (TONs up to 44,000).
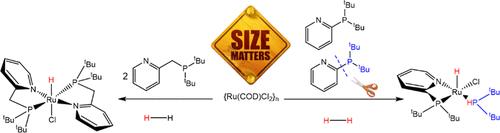
P,N 配体支持的钌配合物中通过氢化作用裂解 C-P 键
研究人员探索了 2-((二叔丁基膦烷基)甲基)吡啶(L1)和 2-(二叔丁基膦烷基)吡啶(L2)支持的氢化钌配合物的反应性。在碱和 10 bar H2 的存在下,{Ru(COD)Cl2}x 与 L1 反应生成预期的复合物 [Ru(L1)2(H)Cl],即 1,而与 L2 反应生成 [Ru(L2)(P(H)tBu2)(H)Cl],即 2,它是 C-P 键裂解的结果。我们能够确定,在反应条件下形成的第一个物种是[Ru(L2)2(H)Cl],3,该物种分解后得到复合物 2,并与[Ru(L2)2Cl2],4 处于平衡状态。根据 DFT 得出的拟议机理,碳的质子化是能量最高的步骤(38.9 kcal/mol),与缓慢反应一致。初步研究表明,配合物 2 在苯甲醛氢化过程中是一种非常活跃的催化剂(吨当量高达 44,000)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


