Therapeutic induction of ferroptosis in tumors using PD-L1 targeting antibody nanogel conjugates
IF 6.6
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Although programmed cell death ligand 1 (PD-L1) is best known for its role in immune suppression, tumor-intrinsic functions are emerging. Here, we report that tumor cells that express PD-L1 are sensitive to ferroptosis inducers such as imidazole ketone erastin (IKE). PD-L1 promotes ferroptosis sensitivity because it suppresses SLC7A11 expression and diminishes glutathione levels. Although the use of anti-PD-L1 antibody drug conjugates (ADCs) could be effective for the delivery of ferroptosis inducers to specific tumor populations, the chemistry of most ferroptosis inducers precludes their incorporation in ADCs. To overcome this challenge, we synthesized an antibody nanogel conjugate (ANC) comprised of an anti-PD-L1 antibody conjugated to a nanogel encapsulated with IKE. This ANC targets PD-L1-expressing cells in vitro and in vivo and induces ferroptosis, resulting in tumor suppression. Importantly, this approach is superior to systemic administration of IKE because it enables enhanced delivery of IKE specifically to tumor cells and it requires lower drug doses for efficacy.
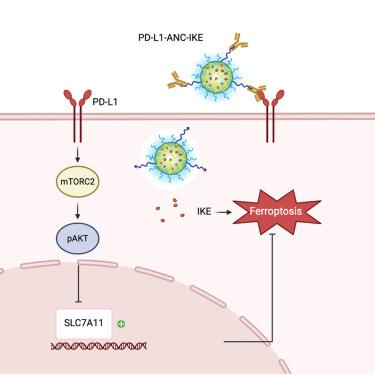

利用 PD-L1 靶向抗体纳米凝胶共轭物治疗诱导肿瘤中的铁变态反应
尽管程序性细胞死亡配体 1(PD-L1)因其在免疫抑制中的作用而广为人知,但其肿瘤内在功能也在不断涌现。在这里,我们报告了表达 PD-L1 的肿瘤细胞对铁变态反应诱导剂(如咪唑酮依拉斯汀(IKE))的敏感性。PD-L1 可抑制 SLC7A11 的表达并降低谷胱甘肽的水平,从而促进铁中毒敏感性。虽然使用抗 PD-L1 抗体药物共轭物(ADCs)可以有效地将铁沉降诱导剂递送到特定的肿瘤人群,但大多数铁沉降诱导剂的化学性质使其无法加入 ADCs。为了克服这一难题,我们合成了一种抗体纳米凝胶共轭物(ANC),它由抗 PD-L1 抗体与包裹有 IKE 的纳米凝胶组成。这种 ANC 可在体外和体内靶向表达 PD-L1 的细胞,并诱导铁凋亡,从而抑制肿瘤。重要的是,这种方法优于全身给药 IKE,因为它能增强 IKE 对肿瘤细胞的特异性递送,而且需要较低的药物剂量才能产生疗效。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Chemical Biology
Biochemistry, Genetics and Molecular Biology-Molecular Medicine
CiteScore
14.70
自引率
2.30%
发文量
143
期刊介绍:
Cell Chemical Biology, a Cell Press journal established in 1994 as Chemistry & Biology, focuses on publishing crucial advances in chemical biology research with broad appeal to our diverse community, spanning basic scientists to clinicians. Pioneering investigations at the chemistry-biology interface, the journal fosters collaboration between these disciplines. We encourage submissions providing significant conceptual advancements of broad interest across chemical, biological, clinical, and related fields. Particularly sought are articles utilizing chemical tools to perturb, visualize, and measure biological systems, offering unique insights into molecular mechanisms, disease biology, and therapeutics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


