Amphiphilic salts as single-component, solvent-free, lithium electrolytes
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
Electrolytes with enhanced thermal stability are sought for next-generation lithium batteries. In this work, we discuss the synthesis, thermal properties, morphology, and ionic conductivity of single-component, solvent-free electrolytes composed of lithium salts with amphiphilic anions. These salts exhibit nanoscale phase segregation between the ionic domains and aliphatic tails of the amphiphilic anions. It is found that for a series of lithium salts with a decane tail, the ionic conductivity is correlated with ion pair binding energy. The ionic conductivity is highest for the decane tailed salt with the –sulfonyl(trifluoromethanesulfonyl)imide head group (LiC10TFSI) at 5.6 × 10–7 S/cm at 70 °C, with salts with – sulfonyl(phenylsulfonyl)imide and –sulfonylazanide anions exhibiting lower conductivity. A salt with an octadecane tail and TFSI headgroup (LiC18TFSI) has further improved ionic conductivity, 4 to 1000 times higher depending on the temperature and 3.6 × 10–5 S/cm at 70 °C. LiC18TFSI is a smectic ionic liquid crystal at intermediate temperatures as confirmed through X-ray scattering experiments and molecular dynamics simulations, whereas LiC10TFSI is suspected to be a disordered ionic liquid at the measurement conditions, highlighting the importance of ionic aggregate morphology on bulk ionic conductivity of electrolytes with ion clusters.
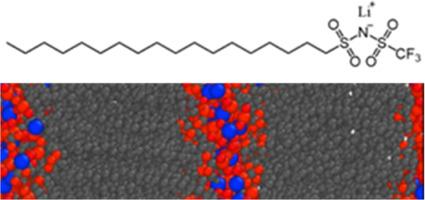
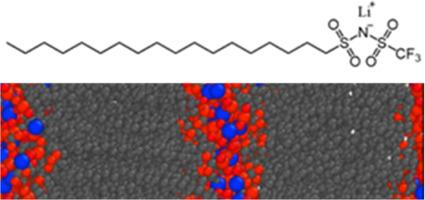
作为单组分、无溶剂锂电解质的两性盐
下一代锂电池需要热稳定性更强的电解质。在这项工作中,我们讨论了由带有两亲阴离子的锂盐组成的单组分无溶剂电解质的合成、热特性、形态和离子导电性。这些盐的离子域和两亲阴离子的脂肪族尾部之间呈现出纳米级的相分离。研究发现,对于一系列具有癸烷尾部的锂盐,离子电导率与离子对结合能相关。在 70°C 时,带有-磺酰基(三氟甲烷磺酰基)酰亚胺头基的癸烷尾盐(LiC10TFSI)的离子电导率最高,为 5.6 × 10-7 S/cm,而带有-磺酰基(苯基磺酰基)酰亚胺和-磺酰氮阴离子的盐的电导率较低。带有十八烷尾部和 TFSI 头基的盐(LiC18TFSI)进一步提高了离子电导率,根据温度的不同可提高 10 到 1000 倍,在 70°C 时为 3.6 × 10-5 S/cm。通过 X 射线散射实验和分子动力学模拟证实,LiC18TFSI 在中间温度下是一种胶凝离子液晶,而在测量条件下,LiC10TFSI 被怀疑是一种无序离子液体,这凸显了离子聚集体形态对带有离子团簇的电解质的体离子电导率的重要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


