Macrophages and nociceptor neurons form a sentinel unit around fenestrated capillaries to defend the synovium from circulating immune challenge
IF 27.7
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
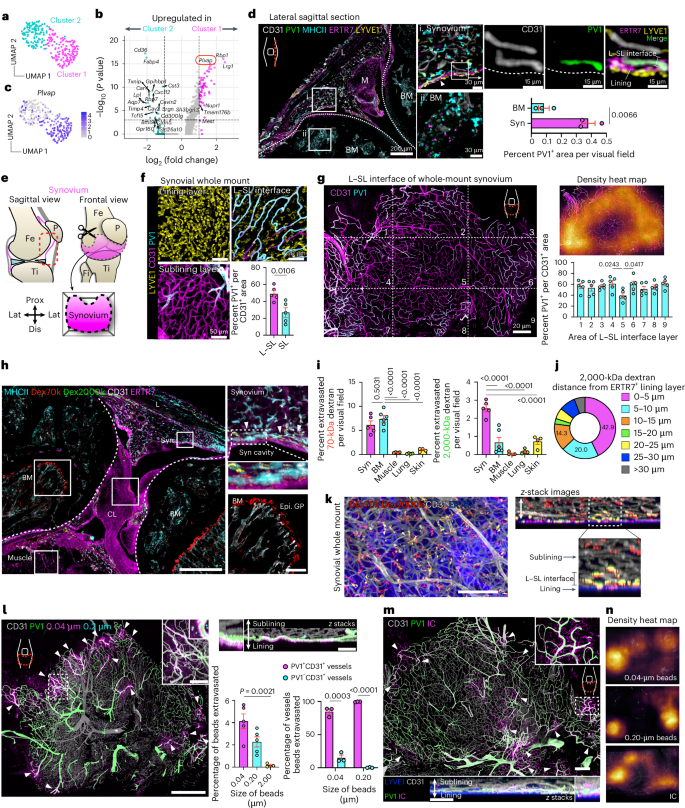
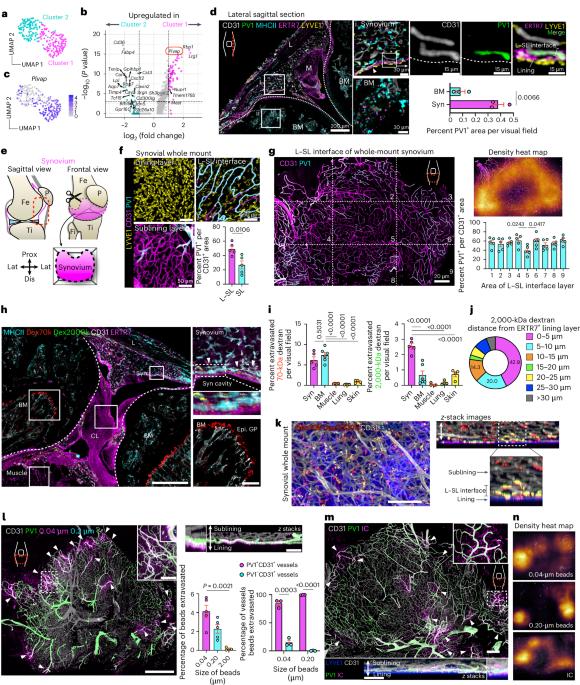
A wide variety of systemic pathologies, including infectious and autoimmune diseases, are accompanied by joint pain or inflammation, often mediated by circulating immune complexes (ICs). How such stimuli access joints and trigger inflammation is unclear. Whole-mount synovial imaging revealed PV1+ fenestrated capillaries at the periphery of the synovium in the lining–sublining interface. Circulating ICs extravasated from these PV1+ capillaries, and nociceptor neurons and three distinct macrophage subsets formed a sentinel unit around them. Macrophages showed subset-specific responses to systemic IC challenge; LYVE1+CX3CR1+ macrophages orchestrated neutrophil recruitment and activated calcitonin gene-related peptide+ (CGRP+) nociceptor neurons via interleukin-1β. In contrast, major histocompatibility complex class II+CD11c+ (MHCII+CD11c+) and MHCII+CD11c– interstitial macrophages formed tight clusters around PV1+ capillaries in response to systemic immune stimuli, a feature enhanced by nociceptor-derived CGRP. Altogether, we identify the anatomical location of synovial PV1+ capillaries and subset-specific macrophage–nociceptor cross-talk that forms a blood–joint barrier protecting the synovium from circulating immune challenges. Why joints are highly responsive to systemic inflammation is unknown. Hasegawa et al. sought to address this question, developing a whole-mount imaging system of the entire synovium to profile the vascular, neuronal and immune components.
巨噬细胞和痛觉神经元在栅栏状毛细血管周围形成一个哨兵单位,保护滑膜免受循环免疫的挑战
包括感染性疾病和自身免疫性疾病在内的多种全身性疾病都伴有关节疼痛或炎症,通常由循环免疫复合物(IC)介导。目前还不清楚这些刺激是如何进入关节并引发炎症的。整块滑膜成像显示,滑膜外围的衬里-衬底界面上有PV1+栅栏状毛细血管。循环 IC 从这些 PV1+ 毛细血管中渗出,痛觉神经元和三个不同的巨噬细胞亚群在其周围形成了一个哨兵单位。巨噬细胞对全身性 IC 挑战表现出亚群特异性反应;LYVE1+CX3CR1+巨噬细胞协调中性粒细胞的招募,并通过白细胞介素-1β激活降钙素基因相关肽+(CGRP+)痛觉感受器神经元。与此相反,主要组织相容性复合体 II+CD11c+ (MHCII+CD11c+)和 MHCII+CD11c- 间质巨噬细胞在全身免疫刺激下在 PV1+ 毛细血管周围形成紧密的集群,这一特征在源于痛觉感受器的 CGRP 的作用下得到增强。总之,我们确定了滑膜 PV1+ 毛细血管的解剖位置以及巨噬细胞-痛觉感受器交叉对话的亚群特异性,这种交叉对话形成了保护滑膜免受循环免疫挑战的血液-关节屏障。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


