Manipulating Backbone Planarity of Ester Functionalized Conjugated Polymer Constitutional Isomer Derivatives Blended with Molecular Acceptors for Controlling Photovoltaic Properties
IF 7
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
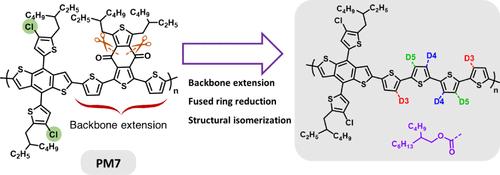
Exploring both electron donor and acceptor phase components in bulk heterojunction structures has contributed to the advancement of organic photovoltaics (OPV) realizing power conversion efficiencies reaching 20%. Being able to control backbone planarity of the donor polymer, while understanding its effects on the polymer conformation and photophysical properties, fosters the groundwork for further achievements in this realm. In this report, three isomeric PM7 derivatives are designed and synthesized where the benzodithiophene-4,8-dione structure is replaced by a quaterthiophene bridge carrying two ester moieties. The placement of these two ester groups varies among three configurational isomers, which ultimately influences the chain conformations and aggregation behavior of each polymer. Specifically, PM7-D3 has ester groups attached to the inner positions of the outer thiophenes showing moderate solution aggregation; PM7-D4 has ester groups attached to the inner positions of the inner thiophenes featuring a twisted backbone with no solution aggregation behavior; and PM7-D5 has ester groups attached to the outer positions of the inner thiophenes with strong solution aggregation. PM7-D5 shows the highest average power conversion efficiency of 11.4% paired with the molecular acceptor L8-BO. In addition, the differences among the polymer backbones are expressed by their state energies and carrier mobility in the corresponding fabricated OPV devices.
操纵与分子受体混合的酯官能化共轭聚合物异构体衍生物的骨架平面度以控制光伏特性
对体异质结结构中电子供体和受体相成分的探索,推动了有机光伏技术(OPV)的发展,使其功率转换效率达到 20%。能够控制供体聚合物的骨架平面度,同时了解其对聚合物构象和光物理性质的影响,为在这一领域取得更大成就奠定了基础。本报告设计并合成了三种异构 PM7 衍生物,其中苯并二噻吩-4,8-二酮结构被带有两个酯分子的四噻吩桥所取代。这两个酯基的位置在三种构型异构体中各不相同,最终影响了每种聚合物的链构象和聚集行为。具体来说,PM7-D3 的酯基附着在外层噻吩的内侧位置,显示出适度的溶液聚集性;PM7-D4 的酯基附着在内层噻吩的内侧位置,具有扭曲的骨架,没有溶液聚集行为;PM7-D5 的酯基附着在内层噻吩的外侧位置,具有强烈的溶液聚集性。PM7-D5 与分子受体 L8-BO 配对后显示出最高的平均功率转换效率(11.4%)。此外,聚合物骨架之间的差异还表现在它们在相应制造的 OPV 器件中的状态能量和载流子迁移率上。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


