Developing Robust Ceria-Supported Catalysts for Catalytic NO Reduction and CO/Hydrocarbon Oxidation
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
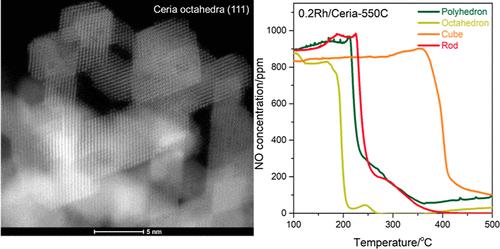
Synthesis of robust and hydrothermally stable PGM/ceria materials for NO, CO, and hydrocarbon abatement remains a formidable challenge, as ceria and PGMs are known to sinter severely >800 °C under hydrothermal conditions, leading to irreversible activity loss. Herein, we tackle this challenge by synthesizing well-defined catalysts with atomically dispersed rhodium supported on ceria with varying abundance of (100), (101), and (111) facets. Evaluation of these catalysts for NO reduction by CO as well as CO and propylene oxidation under model and industrially relevant conditions reveals pronounced reactivity and stability differences. Different modes of interaction of Rh ions with the ceria facets and their facile reducibility were shown to be the crucial parameters controlling reactivity, resulting in pronounced activity and stability variations. Facet-dependent poisoning of surfaces by nitrites was identified as the main reason for deactivation of the catalysts at low temperature, which is mitigated for (111) ceria facets. (111)-enriched ceria nanoparticles survive very harsh hydrothermal aging at 950 °C by maintaining and preserving (111) facets, unlike other ceria nanoparticles which sinter into poorly defined shapes. Thus, putting atomically dispersed PGM sites on (111) ceria facets lead to the catalytic material with the highest activity and stability for all studied reactions, providing the pathway to catalysts that can endure extremely harsh hydrothermal aging conditions.
开发用于催化氮氧化物还原和一氧化碳/碳氢化合物氧化的强效铈支撑催化剂
众所周知,在水热条件下,铈和 PGM 会在 800 °C 下发生严重烧结,从而导致不可逆的活性损失,因此合成用于减少氮氧化物、一氧化碳和碳氢化合物的坚固且水热稳定的 PGM/铈材料仍然是一项艰巨的挑战。在本文中,我们通过在具有不同丰度的 (100)、(101) 和 (111) 面的铈上合成具有原子分散铑支撑的定义明确的催化剂来应对这一挑战。在模型条件和工业相关条件下,对这些催化剂进行一氧化碳还原一氧化氮以及一氧化碳和丙烯氧化的评估发现,其反应活性和稳定性存在明显差异。研究表明,Rh 离子与陶瓷刻面的不同相互作用模式及其易还原性是控制反应活性的关键参数,从而导致了明显的活性和稳定性差异。亚硝酸盐对表面的毒害是催化剂在低温下失活的主要原因,而(111)铈面则减轻了这种毒害。(111)富集的纳米铈颗粒通过保持和保留(111)铈面,在 950 °C 的苛刻水热老化条件下得以存活,而其他纳米铈颗粒则不同,它们会烧结成不明确的形状。因此,将原子分散的 PGM 位点置于(111)铈面上,可使催化材料在所有研究反应中具有最高的活性和稳定性,从而为开发可承受极端苛刻水热老化条件的催化剂提供了途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


