Highly selective capture of lead(II) by a barium-zinc-antimony-oxo cluster-based material: Ion-exchange pathway with single-crystal-to-single-crystal structural transformation
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Efficient removal of Pb2+ from wastewater is of great significance for human health and environmental protection. However, Pb2+ capture from complex wastewater is still very challenging due to the strong competition with other metal ions. Herein, the highly selective removal of Pb2+ has been achieved by a cluster-based material, namely H1.03Ba2.485(H2O)15[Zn2Sb12(μ3-O)8(μ4-O)3(tta)6]·8H2O (H4tta = tartaric acid) (FJSM-BZA). FJSM-BZA exhibits high adsorption capacity (199.29 ± 5.86 mg/g) and fast kinetic response (kinetic equilibrium is reached in 20 min) for Pb2+. In particular, even in the presence of competing ions Mn2+, Co2+, Ni2+, Cu2+, and Cd2+, FJSM-BZA maintains excellent selectivity for Pb2+ with removal rate much higher than that of other metal ions. The material after Pb2+ adsorption could be regenerated by a simple elution method. The unprecedented single-crystal-to-single-crystal (SC to SC) structural transformations during Pb2+ adsorption and elution are unveiled, which helps directly elucidate the distinctive mechanisms of ion exchange between Ba2+ and Pb2+. It is revealed that the strong coordination interactions of the active carboxyl groups at the periphery of the cluster with Pb2+ ions are the intrinsic driving forces for the ion-exchange. This work highlights the design and synthesis of crystalline metal-oxo cluster-based materials for highly selective removal of Pb2+.
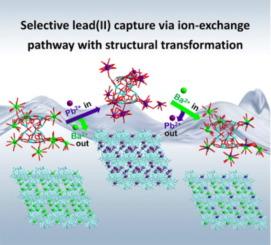
钡锌锑氧团簇材料对铅(II)的高选择性捕获:单晶到单晶结构转变的离子交换途径
有效去除废水中的 Pb2+ 对人类健康和环境保护具有重要意义。然而,由于与其他金属离子的激烈竞争,从复杂废水中捕获 Pb2+ 仍然非常具有挑战性。在此,一种基于团簇的材料,即 H1.03Ba2.485(H2O)15[Zn2Sb12(μ3-O)8(μ4-O)3(ta)6]-8H2O(H4tta = 酒石酸)(FJSM-BZA),实现了对 Pb2+ 的高选择性去除。FJSM-BZA 对 Pb2+ 具有很高的吸附容量(199.29 ± 5.86 mg/g)和快速的动力学响应(20 分钟内达到动力学平衡)。特别是,即使存在竞争离子 Mn2+、Co2+、Ni2+、Cu2+ 和 Cd2+,FJSM-BZA 对 Pb2+ 也能保持极佳的选择性,去除率远高于其他金属离子。吸附 Pb2+ 后的材料可通过简单的洗脱方法再生。在 Pb2+ 吸附和洗脱过程中,该材料发生了前所未有的单晶到单晶(SC 到 SC)的结构转变,这有助于直接阐明 Ba2+ 和 Pb2+ 离子交换的独特机制。研究表明,簇外围活性羧基与 Pb2+ 离子之间的强烈配位相互作用是离子交换的内在驱动力。这项工作突出了设计和合成用于高选择性去除 Pb2+ 的基于结晶金属氧簇的材料。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


