Enhanced co-production of H2 and formic acid via Ni-facilitated Cu+/Cu2+ cycling in an industry-level hybrid water splitting system
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Replacing the oxygen evolution reaction (OER) with the glucose oxidation reaction (GOR) to produce formic acid (FA) is a promising development in green hydrogen production. However, at high current densities, competition with the OER reduces the Faradaic efficiency (FE) of the GOR, limiting its practical applicability and compromising safety. In this study, NiO/CuO nanocomposites grown on Ni foam (NiCu-O/NF) were prepared by oxidizing NiCu-OH/NF to produce a GOR electrocatalyst. The incorporation of Cu effectively suppressed the OER. Additionally, Cu2+ in CuO can spontaneously oxidize glucose, generating Cu2O. However, the resulting Cu2O fails to sustain a high-rate GOR. Owing to the higher surface work function of NiO than that of Cu2O, high-valence Ni species facilitated the reconversion of Cu2+, as evidenced by characterization before and after the GOR. The robust Cu+/Cu2+ cycling enhanced the GOR catalytic performance and ensured good catalytic stability over 40 h. A potential pathway was proposed for producing FA by the GOR. A two-electrode electrolyzer assembled with Ni1Cu2-O/NF (anode) and Ni1Cu2-OH/NF (cathode) afforded 100 and 600 mA cm−2 at 1.46 and 1.82 V, respectively, without any OER interference. Furthermore, FA was confirmed to be the primary anodic product, achieving a maximum FE of 92.67 % at 1.3 V.
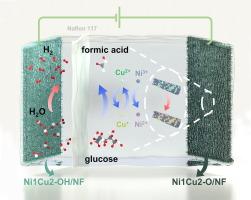
在工业级混合水分离系统中,通过镍促进的 Cu+/Cu2+ 循环提高 H2 和甲酸的共生能力
用葡萄糖氧化反应(GOR)取代氧进化反应(OER)来生产甲酸(FA),是绿色制氢的一个有前途的发展方向。然而,在高电流密度下,与氧进化反应的竞争会降低 GOR 的法拉第效率 (FE),从而限制其实际应用性并影响安全性。本研究通过氧化 NiCu-OH/NF,制备了生长在镍泡沫上的 NiO/CuO 纳米复合材料(NiCu-O/NF),从而生产出一种 GOR 电催化剂。铜的加入有效地抑制了 OER。此外,CuO 中的 Cu2+ 可自发氧化葡萄糖,生成 Cu2O。然而,生成的 Cu2O 无法维持高速率的 GOR。由于 NiO 的表面功函数比 Cu2O 高,高价镍物种促进了 Cu2+ 的再转化,GOR 前后的表征证明了这一点。稳健的 Cu+/Cu2+ 循环提高了 GOR 的催化性能,并确保了 40 小时内良好的催化稳定性。提出了利用 GOR 生产 FA 的潜在途径。用 Ni1Cu2-O/NF(阳极)和 Ni1Cu2-OH/NF(阴极)组装的双电极电解槽在 1.46 和 1.82 V 电压下分别产生了 100 和 600 mA cm-2,没有任何 OER 干扰。此外,FA 被证实是主要的阳极产物,在 1.3 V 时的最大 FE 为 92.67%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


