Striatum-enriched protein, arginase 2 localizes to medium spiny neurons and controls striatal metabolic profile
IF 4.4
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Arginase 2 (Arg2) is the predominant arginase isoenzyme in the brain, however its distribution appears to be limited to selected, region-specific subpopulations of cells. Although striatum is highly enriched with Arg2, precise localization and function of striatal Arg2 have never been studied. Here, we confirm that Arg2 is the only arginase isoenzyme in the striatum, and, using genetic model of total Arg2 loss, we show that Arg2 in this region is fully responsible for arginase catalytic activity, and its loss doesn't induce compensatory activation of Arg1. We exhibit that Arg2 is present in medium spiny neurons (MSNs), striatum-specific projecting neurons, where it localizes in soma and neuronal processes, and is absent in astrocytes or microglia. Finally, analysis of NMR spectroscopy-measured metabolic profiles of striata of Arg2-null mice enabled to recognize two metabolites (NADH and malonic acid) to be significantly altered compared to control animals. Multivariate comparison of the data using orthogonal projections to latent structures discriminant analysis, allowed for discrimination between control and Arg2-null mice and identified metabolites that contributed the most to this between-group dissimilarity. Our study reveals for the first time the localization of Arg2 in MSNs and demonstrates significant role of this enzyme in regulating striatal metabolism. These findings may be especially interesting in the context of Huntington's disease (HD), a disorder that specifically affects MSNs and in which, with the use of mouse models, the onset of pathological phenotypes was recently shown to be preceded by progressive impairment of striatal Arg2, a phenomenon of an unknown significance for disease pathogenesis.
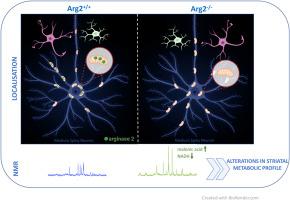
纹状体富集蛋白精氨酸酶 2 定位于中刺神经元并控制纹状体的新陈代谢。
精氨酸酶 2(Arg2)是大脑中最主要的精氨酸酶同工酶,但其分布似乎仅限于特定区域的细胞亚群。虽然纹状体(striatum)高度富集 Arg2,但纹状体 Arg2 的精确定位和功能却从未被研究过。在这里,我们证实 Arg2 是纹状体中唯一的精氨酸酶同工酶,并利用 Arg2 完全缺失的遗传模型,证明该区域的 Arg2 完全负责精氨酸酶的催化活性,其缺失不会引起 Arg1 的代偿性激活。我们发现 Arg2 存在于中棘神经元(MSNs)、纹状体特异性投射神经元中,定位于体和神经元过程,而不存在于星形胶质细胞或小胶质细胞中。最后,通过对 Arg2 缺失小鼠纹状体的核磁共振光谱测量代谢谱进行分析,发现与对照组动物相比,两种代谢物(NADH 和丙二酸)发生了显著变化。利用正交投影潜结构判别分析对数据进行多变量比较,可以区分对照组小鼠和 Arg2-无效小鼠,并确定对组间差异贡献最大的代谢物。我们的研究首次揭示了 Arg2 在 MSN 中的定位,并证明了这种酶在调节纹状体代谢中的重要作用。亨廷顿氏病(Huntington's disease,HD)是一种专门影响 MSN 的疾病,最近通过使用小鼠模型显示,病理表型的出现先于纹状体 Arg2 的进行性损伤,这一现象对疾病发病机制的意义尚不清楚。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Neurochemistry international
医学-神经科学
CiteScore
8.40
自引率
2.40%
发文量
128
审稿时长
37 days
期刊介绍:
Neurochemistry International is devoted to the rapid publication of outstanding original articles and timely reviews in neurochemistry. Manuscripts on a broad range of topics will be considered, including molecular and cellular neurochemistry, neuropharmacology and genetic aspects of CNS function, neuroimmunology, metabolism as well as the neurochemistry of neurological and psychiatric disorders of the CNS.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


