Brain Morphometric Alterations in Focal to Bilateral Tonic–Clonic Seizures in Epilepsy Associated With Excitatory/Inhibitory Imbalance
Abstract
Background
Focal to bilateral tonic–clonic seizures (FBTCS) represent the most severe seizure type in temporal lobe epilepsy (TLE), associated with extensive network abnormalities. Nevertheless, the genetic and cellular factors predispose specific TLE patients to FBTCS remain poorly understood. This study aimed to elucidate the relationship between brain morphometric alterations and transcriptional profiles in TLE patients with FBTCS (FBTCS+) compared to those without FBTCS (FBTCS−).
Methods
We enrolled 126 unilateral TLE patients (89 FBTCS+ and 37 FBTCS−) along with 60 age- and gender-matched healthy controls (HC). We assessed gray matter volume to identify morphometric differences between patients and HC. Partial least squares regression was employed to investigate the association between the morphometric disparities and human brain transcriptomic data obtained from the Allen Human Brain Atlas.
Results
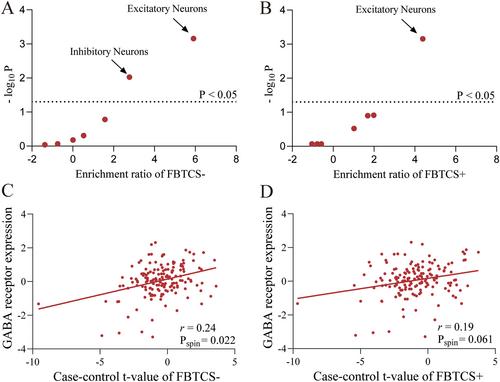
Compared with HC, FBTCS+ patients exhibited morphometric alterations in bilateral cortical and subcortical regions. Conversely, FBTCS− patients exhibited more localized alterations. Imaging transcriptomic analysis revealed both FBTCS− and FBTCS+ groups harbored genes that spatially correlated with morphometric alterations. Additionally, pathway enrichment analysis identified common pathways involved in neural development and synaptic function in both groups. The FBTCS− group displayed unique pathway enrichment in catabolic processes. Furthermore, mapping these genes to specific cell types indicated enrichment in excitatory and inhibitory neurons in the FBTCS− group, while FBTCS+ group only enriched in excitatory neurons. The distinct cellular expression differences between FBTCS− and FBTCS+ groups are consistent with the distribution patterns of GABAergic expression.
Conclusion
We applied imaging transcriptomic analysis linking the morphometric changes and neurobiology in TLE patients with and without FBTCS, including gene expression, biological pathways, cell types, and neurotransmitter receptors. Our findings revealed abnormalities in inhibitory neurons and altered distribution patterns of GABAergic receptors in FBTCS+, suggesting that an excitatory/inhibitory imbalance may contribute to the increased susceptibility of certain individuals to FBTCS.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


