Enhanced and rapid remediation of chlorpyrifos from aqueous phase by amine modified mesoporous silica SBA-15: A systematic investigation on sorption kinetics and mechanistic studies
IF 4.8
3区 材料科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
Pesticides have become a necessary remedy of contemporary agricultural methods. However, the release of excess pesticides to the water bodies is a growing concern worldwide. Chlorpyrifos (CP), an organophosphate based pesticide used for nearly 50,000 tonnes per year globally, has significantly affected the ecosystem. Herein, we have reported that (3-Aminopropyl)triethoxysilane functionalized SBA-15 (ASBA-15) as an efficient adsorbent to remove CP from its aqueous solution to an extent of 1814 mg g−1 within 20 min. The uptake behavior was studied in detail as a function of adsorbent dosage, contact time, and concentration levels of CP. The adsorption findings were well-fitted into the Langmuir isotherm model and pseudo-second-order kinetics. The estimated values of ΔH0 and ΔS0 revealed the exothermic and spontaneous nature of the process. The XRD reflections between 2 = 0.90–1.8o, surface area in the ranges of 781–435 m2 g−1 and pore diameters between 7.2 and 6.0nm indicated the mesoporous as well as stable structure for SBA-15 and ASBA-15 materials at different stages of studies. FT IR studies evidenced the presence of the ‒NH group at 1541 cm−1 in ASBA-15. Thermogravimetric analysis results supported the grafting of the ‒NH group onto ASBA-15 as well as the efficient adsorption of CP. The appearance of characteristic vibration of CP at 1513 cm−1 in CP@ASBA supported the entrapment of CP within ASBA-15. The adsorption and desorption of CP by ASBA-15 were accomplished for five cycles without significant mass loss, which demonstrated the reusability, stability and reversible nature of the material.
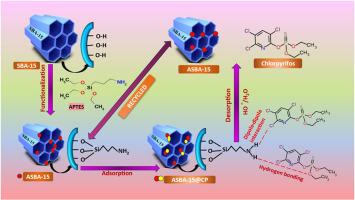
胺改性介孔二氧化硅 SBA-15 增强并快速修复水相中的毒死蜱:对吸附动力学和机理研究的系统调查
杀虫剂已成为当代农业方法的必要补救措施。然而,过量杀虫剂释放到水体中的问题日益引起全世界的关注。毒死蜱(CP)是一种有机磷农药,全球每年使用量近 50,000 吨,对生态系统造成了严重影响。在本文中,我们研究了(3-氨基丙基)三乙氧基硅烷功能化 SBA-15 (ASBA-15)作为一种高效吸附剂,可在 20 分钟内将 CP 从其水溶液中去除至 1814 mg g-1 的程度。详细研究了吸附剂用量、接触时间和 CP 浓度水平对吸附行为的影响。吸附结果与 Langmuir 等温线模型和假二阶动力学十分吻合。ΔH0和ΔS0的估计值显示了吸附过程的放热和自发性质。XRD 反射率在 2 θ = 0.90-1.8o 之间,表面积在 781-435 m2 g-1 之间,孔径在 7.2-6.0nm 之间,这表明 SBA-15 和 ASBA-15 材料在不同研究阶段具有介孔和稳定结构。傅立叶变换红外研究表明,ASBA-15 在 1541 cm-1 处存在 -NH 基团。热重分析结果表明,-NH 基团接枝到了 ASBA-15 上,并有效地吸附了 CP。CP@ASBA 中 CP 在 1513 cm-1 处出现的特征振动证明了 CP 在 ASBA-15 中的吸附作用。CP 在 ASBA-15 上的吸附和解吸循环了五个周期,没有出现明显的质量损失,这证明了该材料的可重复使用性、稳定性和可逆性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Microporous and Mesoporous Materials
化学-材料科学:综合
CiteScore
10.70
自引率
5.80%
发文量
649
审稿时长
26 days
期刊介绍:
Microporous and Mesoporous Materials covers novel and significant aspects of porous solids classified as either microporous (pore size up to 2 nm) or mesoporous (pore size 2 to 50 nm). The porosity should have a specific impact on the material properties or application. Typical examples are zeolites and zeolite-like materials, pillared materials, clathrasils and clathrates, carbon molecular sieves, ordered mesoporous materials, organic/inorganic porous hybrid materials, or porous metal oxides. Both natural and synthetic porous materials are within the scope of the journal.
Topics which are particularly of interest include:
All aspects of natural microporous and mesoporous solids
The synthesis of crystalline or amorphous porous materials
The physico-chemical characterization of microporous and mesoporous solids, especially spectroscopic and microscopic
The modification of microporous and mesoporous solids, for example by ion exchange or solid-state reactions
All topics related to diffusion of mobile species in the pores of microporous and mesoporous materials
Adsorption (and other separation techniques) using microporous or mesoporous adsorbents
Catalysis by microporous and mesoporous materials
Host/guest interactions
Theoretical chemistry and modelling of host/guest interactions
All topics related to the application of microporous and mesoporous materials in industrial catalysis, separation technology, environmental protection, electrochemistry, membranes, sensors, optical devices, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


