Novel polyethylene glycol methyl ether substituted polysiloxane membrane materials with high CO2 permeability and selectivity
IF 5.1
3区 工程技术
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
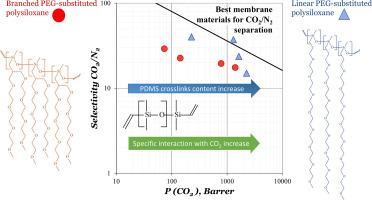
Membrane gas separation is a promising technology for CO2 capture. However, one of the key challenges is the development of stable-in-time and highly permeable materials with increased CO2 permselectivity. In this study, two series of novel polysiloxanes with linear (methyl oligoethyleneglycol allyl ether (PEG8)) and branched (vinyl-bis- [methyltriethyleneglycol] (B-PEG4)) oxygen-containing side substituents were synthesized. By crosslinking PEG-substituted polymethylsiloxanes with polydimethylsiloxane (PDMS), a series of CO2-highly selective membrane materials with a PDMS content of 6.5–50 wt% was obtained for the first time. The effect of the side chain geometry on the sorption and transport properties of these membranes for different gases (N2, O2, CO2, CH4, C2H6) was evaluated. The specific interactions between the PEG substituents and CO2 that contribute to the enhanced selectivity of these materials were identified. The introduction of both linear and branched PEG substituents leads to in an increase in the selectivity of diffusion, solubility, and CO2/gas permeability, compared to PDMS.
The CO2/N2 and CO2/CH4 permselectivities and diffusion selectivities are increases, as well as the permeability coefficients of the membrane materials for all gases are decreases, as the PDMS content reduces from 50 % to 6.5 % wt. Within the PDMS concentrations range between 6.5 and 12.5 % wt., there was a sharp increase in diffusion and permeability coefficients of membrane materials. For this polysiloxanes with linear PEG substituents, an unexpectedly high diffusion selectivity for CO2 was observed. Such value determined the maximum CO2 permselectivity among the polysiloxanes studied. The most promising membrane material from these series was identified as PEG8-substituted polysiloxane with 12.5 wt% PDMS. It has a CO2 permeability coefficient of 1300 Barrer, CO2/N2 selectivity of 37 and CO2/CH4 selectivity of 10.
具有高二氧化碳渗透性和选择性的新型聚乙二醇甲醚替代聚硅氧烷膜材料
膜气体分离是一种前景广阔的二氧化碳捕集技术。然而,关键的挑战之一是开发具有更高的二氧化碳过选择性的实时稳定的高渗透性材料。本研究合成了两个系列的新型聚硅氧烷,分别具有线性(甲基低聚乙二醇烯丙醚(PEG8))和支链(乙烯基-双[甲基三乙二醇](B-PEG4))含氧侧取代基。通过将 PEG 取代的聚甲基硅氧烷与聚二甲基硅氧烷(PDMS)交联,首次获得了一系列 PDMS 含量为 6.5-50 wt%的二氧化碳高选择性膜材料。研究评估了侧链几何形状对这些膜对不同气体(N2、O2、CO2、CH4、C2H6)的吸附和传输特性的影响。确定了 PEG 取代基与二氧化碳之间的特定相互作用,这种相互作用有助于提高这些材料的选择性。与 PDMS 相比,引入线性和支化 PEG 取代基可提高扩散选择性、溶解性和二氧化碳/气体渗透性。在重量百分比为 6.5 至 12.5 % 的 PDMS 浓度范围内,膜材料的扩散系数和渗透系数急剧上升。对于这种带有线性 PEG 取代基的聚硅氧烷,二氧化碳的扩散选择性出乎意料地高。这一数值确定了所研究的聚硅氧烷中最大的二氧化碳过选择性。这些系列中最有前途的膜材料是 PEG8 取代的聚硅氧烷(含 12.5 wt%的 PDMS)。它的二氧化碳渗透系数为 1300 巴,二氧化碳/N2 选择性为 37,二氧化碳/CH4 选择性为 10。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Reactive & Functional Polymers
工程技术-高分子科学
CiteScore
8.90
自引率
5.90%
发文量
259
审稿时长
27 days
期刊介绍:
Reactive & Functional Polymers provides a forum to disseminate original ideas, concepts and developments in the science and technology of polymers with functional groups, which impart specific chemical reactivity or physical, chemical, structural, biological, and pharmacological functionality. The scope covers organic polymers, acting for instance as reagents, catalysts, templates, ion-exchangers, selective sorbents, chelating or antimicrobial agents, drug carriers, sensors, membranes, and hydrogels. This also includes reactive cross-linkable prepolymers and high-performance thermosetting polymers, natural or degradable polymers, conducting polymers, and porous polymers.
Original research articles must contain thorough molecular and material characterization data on synthesis of the above polymers in combination with their applications. Applications include but are not limited to catalysis, water or effluent treatment, separations and recovery, electronics and information storage, energy conversion, encapsulation, or adhesion.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


