Cryptotanshinone alleviates immunosuppression in endometriosis by targeting MDSCs through JAK2/STAT3 pathway
IF 6.7
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Background
Endometriosis (EMS), a well-recognized chronic inflammatory disorder, characterized by significant immune dysregulation, in which myeloid-derived suppressor cells (MDSCs) are essential for facilitating immunosuppression and driving to disease progression. Cryptotanshinone (CTS) is an active compound capable of modulating MDSC-mediated immunosuppression; however, its therapeutic effects and mechanisms in the treatment of EMS remain unclear.
Purpose
This study aims to investigate the therapeutic potential of CTS in modulating MDSCs through JAK2/STAT3 signaling pathway and to evaluate its effects on immune microenvironment and endometriotic lesion growth in EMS.
Methods
Transcriptomic data (GSE141549) and single-cell RNA sequencing data (GSE213216) were analyzed to compare immune cell populations in control endometrium (CE), eutopic endometrium (EuE) and ectopic endometrium (EcE) of patients with EMS. Network pharmacology analysis, surface plasmon resonance (SPR) and cellular thermal shift assay (CETSA) were utilized to explore the molecular mechanism of CTS's effects on MDSCs. A C57BL/6J EMS mice model was established to evaluate CTS's influence on MDSC-mediated immune response in vivo. Flow cytometry and immunofluorescence were used to analyze the immune cell populations, particularly MDSCs and CD8+ T cells. Ex vivo bone marrow (BM)-derived MDSCs were prepared to investigate the modulatory activities of CTS on the frequency and function of MDSCs. The impacts of CTS on JAK2/STAT3 pathway were further examined by western blot.
Results
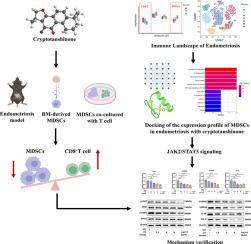
Bioinformatic analysis revealed that, among the three progression stages (CE, EuE, and EcE), the EcE stage exhibited a relatively elevated level of MDSCs and a reduced level of CD8+ T cells. Network pharmacological analysis, along with SPR and CETSA identified that CTS potentially modulates MDSCs in EMS by targeting the JAK2/STAT3 pathway. In vivo studies demonstrated that a relatively high dose of CTS treatment (60mg/kg) effectively inhibited lesion growth, reduced the population of MDSCs, and enhanced CD8+ T cell infiltration. Ex vivo experiments showed that CTS decreased the BM-derived MDSC frequency and rescued the suppressive ability of MDSC upon CD8+ T cells in a dose-dependent manner. Further mechanism analysis confirmed that CTS modulates the expression of immunosuppressive genes and proteins associated with MDSCs through JAK2/STAT3 pathway.
Conclusion
This study is the first to demonstrate that CTS is a promising natural compound for EMS treatment by inhibiting MDSC accumulation and modulating MDSC-mediated immune responses. Its therapeutic efficacy is linked to the modulation of the JAK2/STAT3 signaling pathway.
隐丹参酮通过 JAK2/STAT3 通路靶向 MDSCs 减轻子宫内膜异位症的免疫抑制作用
背景子宫内膜异位症(EMS)是一种公认的慢性炎症性疾病,以严重的免疫失调为特征,其中髓源性抑制细胞(MDSC)是促进免疫抑制和推动疾病进展的关键。本研究旨在探讨隐丹参酮(CTS)通过JAK2/STAT3信号通路调节MDSCs的治疗潜力,并评估其对EMS的免疫微环境和子宫内膜异位病变生长的影响。方法分析转录组数据(GSE141549)和单细胞RNA测序数据(GSE213216),比较EMS患者对照子宫内膜(CE)、异位子宫内膜(EuE)和异位子宫内膜(EcE)的免疫细胞群。研究人员利用网络药理学分析、表面等离子体共振(SPR)和细胞热转移测定(CETSA)来探讨CTS对MDSCs影响的分子机制。建立了 C57BL/6J EMS 小鼠模型,以评估 CTS 对 MDSC 介导的体内免疫反应的影响。流式细胞术和免疫荧光用于分析免疫细胞群,特别是 MDSCs 和 CD8+ T 细胞。制备了体内骨髓(BM)衍生的MDSCs,以研究CTS对MDSCs频率和功能的调节活性。结果生物信息学分析表明,在三个进展阶段(CE、EuE 和 EcE)中,EcE 阶段的 MDSCs 水平相对升高,CD8+ T 细胞水平相对降低。网络药理学分析以及 SPR 和 CETSA 发现,CTS 可通过靶向 JAK2/STAT3 通路调节 EMS 中的 MDSCs。体内研究表明,相对较高的 CTS 治疗剂量(60 毫克/千克)能有效抑制病变的生长,减少 MDSCs 的数量,并增强 CD8+ T 细胞的浸润。体内外实验显示,CTS以剂量依赖的方式降低了BM来源的MDSC频率,并挽救了MDSC对CD8+ T细胞的抑制能力。进一步的机制分析证实,CTS 可通过 JAK2/STAT3 通路调节与 MDSC 相关的免疫抑制基因和蛋白的表达。它的疗效与 JAK2/STAT3 信号通路的调节有关。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Phytomedicine
医学-药学
CiteScore
10.30
自引率
5.10%
发文量
670
审稿时长
91 days
期刊介绍:
Phytomedicine is a therapy-oriented journal that publishes innovative studies on the efficacy, safety, quality, and mechanisms of action of specified plant extracts, phytopharmaceuticals, and their isolated constituents. This includes clinical, pharmacological, pharmacokinetic, and toxicological studies of herbal medicinal products, preparations, and purified compounds with defined and consistent quality, ensuring reproducible pharmacological activity. Founded in 1994, Phytomedicine aims to focus and stimulate research in this field and establish internationally accepted scientific standards for pharmacological studies, proof of clinical efficacy, and safety of phytomedicines.
文献相关原料
公司名称
产品信息
索莱宝
CTS
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


