Mitigation strategies of salicylhydroxamic acid collector with oxalic acid in goethite flotation
IF 5.4
3区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
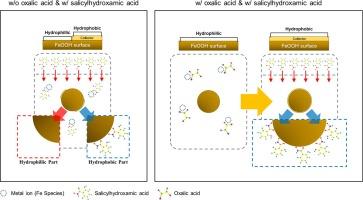
Flotation of goethite, a representative mineral of laterite ore, which is essential source of nickel used in secondary batteries, was conducted using salicylhydroxamic acid as the collector. The presence of ferric ions derived from goethite in the pulp resulted in an increased consumption of the collector during flotation. Following three washing stages, the maximum recovery of 98.5 wt% was achieved with a collector dosage of only 60 % in the absence of a washing stage. The oxalic acid is employed to eliminate ferric ions via precipitation without the requirement for supplementary water, energy, and time-consuming washing stages. The utilization of 40 g⋅t−1 of oxalic acid yielded comparable recovery outcomes to those observed in the three washing stages. Furthermore, theoretical investigations were conducted to determine the collector species influencing goethite flotation with density functional theory. It was observed that compared with that of goethite with non-ionized salicylhydroxamic acid complex, the complexation energy for goethite with ionized salicylhydroxamic acid was lower by 113.9 kJ⋅mol−1, indicating that the optimum adsorption onto the mineral surface strongly depends on ionized salicylhydroxamic acid species. This study presents a potential method for reduction of reagent consumption in the flotation of future complex laterite ore, an acidic soil in which metal ions might be present. Additionally, the theoretical framework elucidates the complexation of collectors to minerals that are challenging to quantify experimentally.
水杨酰羟肟酸捕收剂与草酸在辉锑矿浮选中的缓解策略
水杨羟肟酸是红土矿的一种代表性矿物,是二次电池所用镍的重要来源。由于矿浆中存在来自鹅铁矿的铁离子,导致浮选过程中捕收剂的消耗量增加。经过三个洗涤阶段后,在没有洗涤阶段的情况下,捕收剂用量仅为 60%,就达到了 98.5% 的最高回收率。草酸可通过沉淀消除铁离子,而无需补充水、能源和耗时的洗涤阶段。使用 40 g⋅t-1 的草酸所产生的回收结果与在三个洗涤阶段观察到的结果相当。此外,还利用密度泛函理论进行了理论研究,以确定影响鹅辉石浮选的捕收剂种类。结果表明,与非离子化水杨羟肟酸络合物相比,离子化水杨羟肟酸络合物对鹅卵石的络合能低 113.9 kJ-mol-1,这表明矿物表面的最佳吸附主要取决于离子化水杨羟肟酸物种。这项研究提出了一种潜在的方法,可减少未来复杂红土矿(一种可能存在金属离子的酸性土壤)浮选过程中的试剂消耗。此外,该理论框架还阐明了捕收剂与矿物的络合作用,而这种络合作用很难通过实验进行量化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry Communications
化学-无机化学与核化学
CiteScore
5.50
自引率
7.90%
发文量
1013
审稿时长
53 days
期刊介绍:
Launched in January 1998, Inorganic Chemistry Communications is an international journal dedicated to the rapid publication of short communications in the major areas of inorganic, organometallic and supramolecular chemistry. Topics include synthetic and reaction chemistry, kinetics and mechanisms of reactions, bioinorganic chemistry, photochemistry and the use of metal and organometallic compounds in stoichiometric and catalytic synthesis or organic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


