Bimetallic coordination polymers synthesized from pyrazine dicarboxylic acid serve as efficient electrocatalysts for enhancing the oxygen evolution reaction
IF 4.4
3区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
The pursuit of cost-effective and efficient electrocatalysts for water oxidation is crucial for various applications for the storage and conversion of electrochemical energy. Coordination polymers (CPs) have garnered significant interest as potential electrocatalysts, as their catalytic efficiency can be precisely tuned through the design of coordination layers that boast highly accessible and highly reactive metal sites. However, CP-based catalysts face substantial challenges for their application in electrocatalytic oxidation processes because of their limited activity and poor stability. In this study, we employed a mixed-metal approach to develop CoxNi1-x-PDAs incorporating two functional sites specifically designed for facilitating the oxidation of water. The presence of both Co and Ni enhances electron transport through synergistic effects within the CoxNi1-x-PDAs structure. By adjusting the metal ratios in these coordination polymers, an optimized Co3/4Ni1/4-PDA demonstrated impressive performance in water oxidation under alkaline conditions during oxygen evolution reactions, a current density of 10 mA cm−2 was achieved with an overpotential of 322 mV, along with a Tafel slope measured at 87 mV dec−1. This approach involving mixed metals seeks to exploit the synergistic effects among various metal centers, which could result in efficient electrocatalysts for the oxidation of small molecules. Our findings present a promising avenue for utilizing CPs materials within the realm of electrocatalysis.
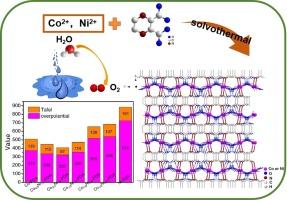
以吡嗪二羧酸为原料合成的双金属配位聚合物可作为高效电催化剂促进氧进化反应
寻求具有成本效益的高效水氧化电催化剂对于电化学能量的储存和转换等各种应用至关重要。配位聚合物(CP)作为潜在的电催化剂引起了人们的极大兴趣,因为通过设计配位层,可以精确地调整它们的催化效率,而配位层则拥有高易得性和高活性的金属位点。然而,由于活性有限且稳定性差,CP 催化剂在电催化氧化过程中的应用面临着巨大挑战。在本研究中,我们采用了一种混合金属方法来开发 CoxNi1-x-PDAs,其中包含了两个专门用于促进水氧化的功能位点。Co 和 Ni 的存在通过 CoxNi1-x-PDAs 结构中的协同效应增强了电子传输。通过调整这些配位聚合物中的金属比例,经过优化的 Co3/4Ni1/4-PDA 在碱性条件下进行氧进化反应时,在水氧化过程中表现出令人印象深刻的性能,电流密度达到 10 mA cm-2,过电位为 322 mV,测得的塔菲尔斜率为 87 mV dec-1。这种涉及混合金属的方法旨在利用各种金属中心之间的协同效应,从而产生高效的小分子氧化电催化剂。我们的研究结果为在电催化领域利用氯化石蜡材料提供了一条前景广阔的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry Communications
化学-无机化学与核化学
CiteScore
5.50
自引率
7.90%
发文量
1013
审稿时长
53 days
期刊介绍:
Launched in January 1998, Inorganic Chemistry Communications is an international journal dedicated to the rapid publication of short communications in the major areas of inorganic, organometallic and supramolecular chemistry. Topics include synthetic and reaction chemistry, kinetics and mechanisms of reactions, bioinorganic chemistry, photochemistry and the use of metal and organometallic compounds in stoichiometric and catalytic synthesis or organic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


