Mechanistic insight into the catalytic activities of metallic sites on nitrogen-doped graphene quantum dots for CO2 hydrogenation
IF 3.1
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
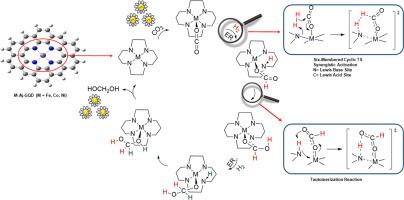
The origin of selectivity and activity of the CO2 hydrogenation reaction on single-atom catalysts composed of three adjacent 3d transition metals (Fe, Co, and Ni) supported on N-doped graphene quantum dots were systematically investigated and compared using density functional theory (DFT) calculations, natural bond orbital (NBO), and quantum theory of atoms in molecules (QTAIM) analysis. This study reveals that π-backbonding between the metal and CO2* does not occur and [CO2]δ+ species drive the reaction. The CO2* reacts with H2 via the Eley-Rideal (ER) mechanism by using the synergistic effects of the N site. The higher the partial positive charge on the C atom, the lower the Ea of the reaction. Subsequently, a tautomerization reaction, which is facilitated by hydrogen bonding, occurs and hydrogen is transferred to HCOO* resulting in the formation of CHOOH*. This study shows the selective formation of formic acid from CO2 is accessible on these SACs and Fe-SAC is the best one between these three catalysts. Although CO2 is more inert than formic acid the H2 molecule reacts with the adsorbed formic acid more difficult than the adsorbed CO2. It is because the hydrogenation of formic acid causes C-H bond formation resulting in failure of the coordinated O atom's octet and the unstable H2COOH* is formed. This step is the rate-determining step of HOCH2OH formation from CO2, with Ea of 1.94, 2.03, and 2.23 eV for Fe, Co, and Ni, respectively. Finally, the system undergoes another tautomerization reaction resulting in the formation of HOCH2OH (formaldehyde monohydrate).
氮掺杂石墨烯量子点上金属位点对二氧化碳加氢催化活性的机理研究
利用密度泛函理论(DFT)计算、天然键轨道(NBO)和分子中原子量子理论(QTAIM)分析,系统地研究和比较了在掺杂 N 的石墨烯量子点上支撑的由三种相邻 3d 过渡金属(Fe、Co 和 Ni)组成的单原子催化剂上进行 CO2 加氢反应的选择性和活性的起源。研究发现,金属与 CO2* 之间不存在π键,[CO2]δ+ 物种驱动反应。利用 N 位点的协同效应,CO2* 通过 Eley-Rideal (ER) 机制与 H2 反应。C 原子上的部分正电荷越高,反应的 Ea 值就越低。随后,在氢键的促进下发生了同分异构反应,氢转移到 HCOO*,形成 CHOOH*。这项研究表明,在这些 SAC 上可以从 CO2 选择性地生成甲酸,而 Fe-SAC 是这三种催化剂中最好的一种。虽然 CO2 比甲酸更惰性,但 H2 分子与吸附的甲酸发生反应比与吸附的 CO2 发生反应更困难。这是因为甲酸的氢化作用会形成 C-H 键,导致配位 O 原子的八位失效,形成不稳定的 H2COOH*。这一步骤是 CO2 形成 HOCH2OH 的速率决定步骤,Fe、Co 和 Ni 的 Ea 分别为 1.94、2.03 和 2.23 eV。最后,系统会发生另一个同分异构反应,形成 HOCH2OH(甲醛一水合物)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Carbon Trends
Materials Science-Materials Science (miscellaneous)
CiteScore
4.60
自引率
0.00%
发文量
88
审稿时长
77 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


