Tyrosine bioconjugation using stably preparable urazole radicals
引用次数: 0
Abstract
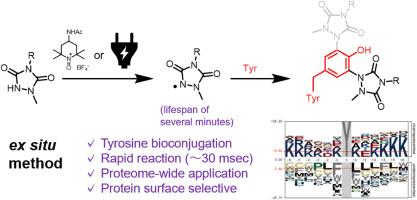
Our ex situ tyrosine bioconjugation method utilizes the oxidation of N-methylurazole by Bobbitt's salt or electrochemical activation to generate stable radicals for selective tyrosine labeling, thereby addressing the longstanding challenges in protein bioconjugation. Unlike traditional tyrosine conjugation techniques that rely on closed-shell chemistry with 1,2,4-triazoline-3,5-dione derivatives, this method introduces an alternative bond-forming mechanism that expands bioconjugation through a radical-based pathway that enhances tyrosine selectivity. The applicability of this method was demonstrated for various proteins and validated through proteome-wide analyses, highlighting its potential as a valuable tool for investigating protein function and interaction dynamics.
利用可稳定制备的脲唑自由基进行酪氨酸生物键合
我们的原位酪氨酸生物共轭方法利用博比特盐或电化学活化作用氧化 N-甲基脲唑,生成稳定的自由基进行选择性酪氨酸标记,从而解决了蛋白质生物共轭领域的长期难题。传统的酪氨酸共轭技术依赖于 1,2,4-三唑啉-3,5-二酮衍生物的闭壳化学反应,与之不同的是,这种方法引入了另一种成键机制,通过基于自由基的途径扩展了生物共轭,从而提高了酪氨酸的选择性。该方法适用于多种蛋白质,并通过全蛋白质组分析进行了验证,凸显了其作为研究蛋白质功能和相互作用动态的重要工具的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


