Microwave-assisted synthesis of pyrrolidinyl-spirooxindoles via tandem 1,3-dipolar cycloaddition and oxidative dehydrogenation
IF 2.1
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
An oxidant-free, microwave-promoted tandem 1,3-dipolar cycloaddition and dehydrogenation oxidation is achieved under environmentally friendly conditions, delivering novel fused pyrrolidinyl-spirooxindoles in good to excellent yields. This work provides powerful means to expand the structural diversity of pyrrolidinyl-spirooxindole as a promising scaffold for novel drug discovery.
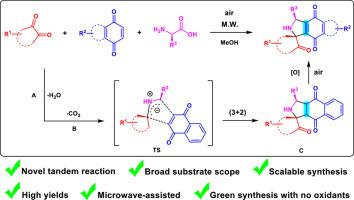
通过串联 1,3-二极环加成和氧化脱氢微波辅助合成吡咯烷基螺氧吲哚
在环境友好的条件下,实现了无氧化剂、微波促进的串联 1,3-二极环加成和脱氢氧化,以良好到极佳的收率获得了新型融合吡咯烷基螺氧吲哚。这项工作为扩大吡咯烷基螺氧吲哚的结构多样性提供了强有力的手段,为新型药物的发现提供了前景广阔的支架。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


