State-of-the-art phosphorylation of water-soluble allyl cellulose via a green radical-mediated “click” reaction pathway with enhanced antifungal activity
IF 10.7
1区 化学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
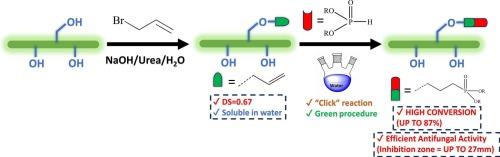
Allyl Cellulose (AC) was synthesized using allyl bromide in sodium hydroxide (NaOH)/urea aqueous solution. By employing a molar ratio of 6:1 of allyl bromide/cellulose, low-degree of substitution (DS) water-soluble AC (AC1) was obtained (DS = 0.67). Then, radical-mediated “click” reactions of AC1 with diethyl phosphite (DEP) and dibutyl phosphite (DBP) were carried out in deionized water as green reaction medium, using 4,4′-azobis(4-cyanovaleric acid) (ACVA) as a radical initiator. The synthesized AC1-DEP and AC1-DBP showed high conversion percentages of 84 % and 87 %, respectively. Fourier-transform infrared spectroscopy (FTIR) and nuclear magnetic resonance (NMR) techniques were used to validate the structure of the products and confirm the successful radical-mediated “click” reactions of AC1 with dialkyl phosphite (DAP) in water. X-ray diffraction (XRD), thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), static contact angle, and scanning electron microscopy with energy dispersive X-ray spectroscopy (SEM/EDX) analyses were used to investigate structural, thermal, and surface properties, revealing an increase in flexibility and hydrophobicity upon phosphorylation. AC1-diethyl phosphite (AC1-DEP) and AC1-dibutyl phosphite (AC1-DBP) exhibited efficient antifungal activities with inhibition zone ranges from 14 to 27 mm.
通过绿色自由基介导的 "点击 "反应途径对水溶性烯丙基纤维素进行最先进的磷酸化处理,增强抗真菌活性
在氢氧化钠(NaOH)/尿素水溶液中使用烯丙基溴合成了烯丙基纤维素(AC)。通过使用 6:1 的烯丙基溴/纤维素摩尔比,得到了低取代度(DS)水溶性 AC(AC1)(DS = 0.67)。然后,以 4,4′-偶氮双(4-氰基戊酸)(ACVA)为自由基引发剂,在去离子水作为绿色反应介质中,进行了 AC1 与亚磷酸二乙酯(DEP)和亚磷酸二丁酯(DBP)的自由基介导 "点击 "反应。合成的 AC1-DEP 和 AC1-DBP 转化率分别高达 84 % 和 87 %。傅立叶变换红外光谱(FTIR)和核磁共振(NMR)技术用于验证产物的结构,并确认 AC1 与亚磷酸二烷基酯(DAP)在水中成功地进行了自由基介导的 "点击 "反应。利用 X 射线衍射 (XRD)、热重分析 (TGA)、差示扫描量热 (DSC)、静态接触角和扫描电子显微镜与能量色散 X 射线光谱 (SEM/EDX) 分析来研究结构、热和表面特性,结果表明磷酸化后柔性和疏水性增加。AC1-二乙基亚磷酸酯(AC1-DEP)和 AC1-二丁基亚磷酸酯(AC1-DBP)表现出高效的抗真菌活性,抑制区范围为 14 至 27 毫米。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Carbohydrate Polymers
化学-高分子科学
CiteScore
22.40
自引率
8.00%
发文量
1286
审稿时长
47 days
期刊介绍:
Carbohydrate Polymers stands as a prominent journal in the glycoscience field, dedicated to exploring and harnessing the potential of polysaccharides with applications spanning bioenergy, bioplastics, biomaterials, biorefining, chemistry, drug delivery, food, health, nanotechnology, packaging, paper, pharmaceuticals, medicine, oil recovery, textiles, tissue engineering, wood, and various aspects of glycoscience.
The journal emphasizes the central role of well-characterized carbohydrate polymers, highlighting their significance as the primary focus rather than a peripheral topic. Each paper must prominently feature at least one named carbohydrate polymer, evident in both citation and title, with a commitment to innovative research that advances scientific knowledge.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


