One–pot green synthesis of TiO2 nanoparticles using Inula Viscosa leaf extract as an efficient photocatalyst for organic dyes removal
IF 4.1
3区 化学
Q2 CHEMISTRY, PHYSICAL
Journal of Photochemistry and Photobiology A-chemistry
Pub Date : 2024-11-16
DOI:10.1016/j.jphotochem.2024.116158
引用次数: 0
Abstract
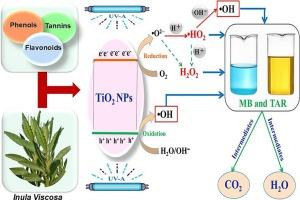
In this research, TiO2 nanoparticles (TiO2-NPs) were prepared via a green synthesis using Inula Viscosa aqueous extract, which has not been stated for the preparation of TiO2-based nanomaterials. Different calcination temperatures (400, 500, and 600 °C) were used to examine their effect on the properties and the photoactivity of TiO2. Multiple characterization methods were employed to assess the different features of the prepared materials. The detected phenolic compounds in the investigated plant through the phytochemical analysis acted as a capping agent that has a vital role in the development of nanosized TiO2 oxide. According to the structural analysis, the obtained nanoparticles have a consistent distribution over the sample surface and are about 9–18 nm in size. Results also confirmed the occurrence of pure anatase phase TiO2 nanoparticles at all calcination temperatures. The photoactivity was analyzed for the degradation of methylene blue and tartrazine organic dyes. TiO2-NPs calcined at 500 °C exhibited the best photocatalytic performances where a complete removal efficiency (100 %) toward both pollutants was achieved in only 60 min of UV irradiation. As a photocatalyst, the synthesized TiO2-NPs outperformed many of the greenly reported nanomaterials in literature.
利用茵陈叶提取物一锅绿色合成 TiO2 纳米粒子,作为去除有机染料的高效光催化剂
本研究采用茵陈水提取物进行绿色合成,制备了二氧化钛纳米颗粒(TiO2-NPs)。采用不同的煅烧温度(400、500 和 600 °C)考察它们对 TiO2 性能和光活性的影响。采用多种表征方法来评估所制备材料的不同特征。通过植物化学分析发现,所研究植物中的酚类化合物是一种封端剂,在纳米二氧化钛氧化物的开发过程中起着至关重要的作用。根据结构分析,获得的纳米颗粒在样品表面分布一致,大小约为 9-18 纳米。结果还证实,在所有煅烧温度下,都出现了纯锐钛矿相二氧化钛纳米粒子。光活性分析了亚甲基蓝和酒石酸有机染料的降解情况。在 500 °C 下煅烧的 TiO2-NPs 表现出最佳的光催化性能,在紫外线照射下,仅 60 分钟就能完全去除这两种污染物(100%)。作为一种光催化剂,合成的 TiO2-NPs 优于文献中报道的许多绿色纳米材料。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.90
自引率
7.00%
发文量
580
审稿时长
48 days
期刊介绍:
JPPA publishes the results of fundamental studies on all aspects of chemical phenomena induced by interactions between light and molecules/matter of all kinds.
All systems capable of being described at the molecular or integrated multimolecular level are appropriate for the journal. This includes all molecular chemical species as well as biomolecular, supramolecular, polymer and other macromolecular systems, as well as solid state photochemistry. In addition, the journal publishes studies of semiconductor and other photoactive organic and inorganic materials, photocatalysis (organic, inorganic, supramolecular and superconductor).
The scope includes condensed and gas phase photochemistry, as well as synchrotron radiation chemistry. A broad range of processes and techniques in photochemistry are covered such as light induced energy, electron and proton transfer; nonlinear photochemical behavior; mechanistic investigation of photochemical reactions and identification of the products of photochemical reactions; quantum yield determinations and measurements of rate constants for primary and secondary photochemical processes; steady-state and time-resolved emission, ultrafast spectroscopic methods, single molecule spectroscopy, time resolved X-ray diffraction, luminescence microscopy, and scattering spectroscopy applied to photochemistry. Papers in emerging and applied areas such as luminescent sensors, electroluminescence, solar energy conversion, atmospheric photochemistry, environmental remediation, and related photocatalytic chemistry are also welcome.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


