High storage capacity and rapid methane hydrate formation using low concentrations of a new surfactant: A mimic of SDS and amino acid scaffold
IF 10.1
1区 工程技术
Q1 ENERGY & FUELS
引用次数: 0
Abstract
The development of efficient, non-foaming promoters is essential for advancing the industrial applications of solidified gas hydrates in carbon capture, natural gas storage, and transportation. In this study, a novel surfactant, containing sulfonate, amide, and carboxyl groups (SSAC), was introduced as a promoter for methane hydrate formation. SSAC was synthesized by integrating the chemistries of amino acids and sodium dodecyl sulfate (SDS), distinguishing it from existing promoters. High-pressure autoclave experiments demonstrated that SSAC significantly enhanced the kinetics of methane hydrate formation, at a low concentration of 5 ppm, achieving a maximum water-to-hydrate conversion of 85.2 %, equivalent to a storage capacity of 163.5 v/v in deionized water. Increasing the SSAC concentration to 500 ppm resulted in an impressive conversion rate of 94.6 % and a storage capacity of 181.6 v/v. Methane recovery was accomplished without foaming within 15 min during hydrate dissociation at room temperature, addressing a critical challenge in current hydrate-based storage systems. Molecular dynamics simulations further revealed that SSAC molecules act as collectors for methane molecules in solution, thereby enhancing the rate of hydrate growth and increasing the number of hydrate cavities. Notably, SSAC exhibited a biodegradation level of 41 % after 28 days, indicating its potential for natural degradation and environmental compatibility. This combination of low concentration efficiency, foam-free formation, environmental sustainability, and enhanced methane collection is unprecedented in the current literature, highlighting the innovative nature of this work. These findings suggest that the integration of amino acid structures with anionic surfactants offers a promising strategy for designing effective promoters, with significant implications for energy storage, seawater desalination, and carbon capture technologies.
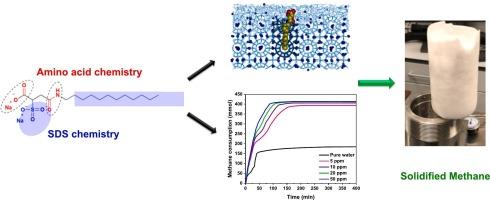
利用低浓度新型表面活性剂:SDS 和氨基酸支架的模拟物,实现高储存能力和甲烷水合物的快速形成
开发高效、不起泡的促进剂对于推动固化天然气水合物在碳捕集、天然气储存和运输领域的工业应用至关重要。本研究引入了一种含有磺酸基、酰胺基和羧基的新型表面活性剂 (SSAC),作为甲烷水合物形成的促进剂。SSAC 是通过整合氨基酸和十二烷基硫酸钠(SDS)的化学成分合成的,有别于现有的促进剂。高压釜实验表明,在 5 ppm 的低浓度下,SSAC 能显著增强甲烷水合物形成的动力学,实现 85.2 % 的最大水-水合物转化率,相当于去离子水中 163.5 v/v 的存储容量。将 SSAC 浓度提高到 500 ppm 后,转化率达到 94.6%,存储容量达到 181.6 v/v。在室温下水合物解离过程中,甲烷在 15 分钟内无泡沫回收,解决了当前基于水合物的存储系统所面临的关键挑战。分子动力学模拟进一步显示,SSAC 分子可作为溶液中甲烷分子的收集器,从而提高水合物的生长速度并增加水合物空腔的数量。值得注意的是,SSAC 在 28 天后的生物降解水平为 41%,这表明它具有自然降解和环境相容性的潜力。这种低浓度效率、无泡沫形成、环境可持续性和增强甲烷收集的组合在目前的文献中是前所未有的,凸显了这项工作的创新性。这些发现表明,氨基酸结构与阴离子表面活性剂的结合为设计有效的促进剂提供了一种前景广阔的策略,对能源储存、海水淡化和碳捕集技术具有重要意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Applied Energy
工程技术-工程:化工
CiteScore
21.20
自引率
10.70%
发文量
1830
审稿时长
41 days
期刊介绍:
Applied Energy serves as a platform for sharing innovations, research, development, and demonstrations in energy conversion, conservation, and sustainable energy systems. The journal covers topics such as optimal energy resource use, environmental pollutant mitigation, and energy process analysis. It welcomes original papers, review articles, technical notes, and letters to the editor. Authors are encouraged to submit manuscripts that bridge the gap between research, development, and implementation. The journal addresses a wide spectrum of topics, including fossil and renewable energy technologies, energy economics, and environmental impacts. Applied Energy also explores modeling and forecasting, conservation strategies, and the social and economic implications of energy policies, including climate change mitigation. It is complemented by the open-access journal Advances in Applied Energy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


