LIFU-unlocked endogenous H2S generation for enhancing atherosclerosis-specific gas-enzymatic therapy
IF 12.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Atherosclerotic plaques, which are characterized by endothelial oxidative stress, lipid metabolism disorders and persistent inflammation, can induce serious cardiovascular diseases. However, the pharmacotherapies currently used to treat atherosclerosis (AS), such as lipid-lowering and antithrombotic drugs, can regulate only a single pathological feature of AS, and there is still a dearth of integrated platforms for the multifaceted regulation of AS progression. Herein, we developed a synergistic combination of endogenous H2S gas therapy with a multienzyme-like nanozyme (named LyP−1Lip@HS) for the treatment of AS. The high affinity of the LyP-1 peptide for macrophages and foam cells within plaques allows LyP−1Lip@HS to actively target atherosclerotic lesions. After cavitation was induced by low-intensity focused ultrasound (LIFU), the lipid membrane of LyP−1Lip@HS was disrupted, thereby "unlocking" the enzyme-like activity of hollow mesoporous Prussian blue (HMPB) and facilitating the release of the endogenous H2S donor S-allyl-L-cysteine (SAC). Notably, H2S endogenously generated by enzymatic catalysis plays multiple roles, upregulating the ATP-binding cassette transporter A1 in foam cells to increase lipid efflux and promote the conversion of M1 macrophages to M2 macrophages. Moreover, the high level of reactive oxygen species in the inflammatory microenvironment of the plaque was mitigated. Overall, LyP−1Lip@HS provides a specific and controlled treatment to prevent oxidative stress, inflammation and lipid metabolism disorders, making it a candidate for AS treatment.
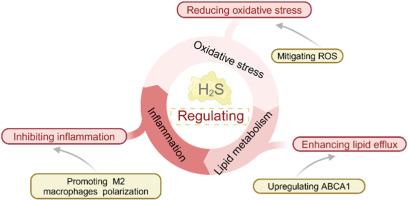
LIFU 解锁内源性 H2S 生成,加强动脉粥样硬化特异性气体酶疗法
动脉粥样硬化斑块以内皮氧化应激、脂质代谢紊乱和持续炎症为特征,可诱发严重的心血管疾病。然而,目前用于治疗动脉粥样硬化(AS)的药物疗法,如降脂药和抗血栓药,只能调控AS的单一病理特征,仍然缺乏从多方面调控AS进展的综合平台。在此,我们开发了内源性H2S气体疗法与多酶样纳米酶(命名为LyP-1Lip@HS)的协同组合来治疗强直性脊柱炎。LyP-1肽对斑块内巨噬细胞和泡沫细胞的高亲和力使LyP-1Lip@HS能主动靶向动脉粥样硬化病变。低强度聚焦超声(LIFU)诱导空化后,LyP-1Lip@HS的脂膜被破坏,从而 "释放 "了中空介孔普鲁士蓝(HMPB)的酶样活性,促进了内源性H2S供体S-烯丙基-L-半胱氨酸(SAC)的释放。值得注意的是,酶催化产生的内源性 H2S 起着多重作用,它能上调泡沫细胞中的 ATP 结合盒转运体 A1,增加脂质外流,促进 M1 巨噬细胞向 M2 巨噬细胞转化。此外,斑块炎症微环境中高水平的活性氧也得到了缓解。总之,LyP-1Lip@HS 提供了一种特异性和可控的治疗方法来预防氧化应激、炎症和脂质代谢紊乱,使其成为治疗强直性脊柱炎的候选药物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biomaterials
工程技术-材料科学:生物材料
CiteScore
26.00
自引率
2.90%
发文量
565
审稿时长
46 days
期刊介绍:
Biomaterials is an international journal covering the science and clinical application of biomaterials. A biomaterial is now defined as a substance that has been engineered to take a form which, alone or as part of a complex system, is used to direct, by control of interactions with components of living systems, the course of any therapeutic or diagnostic procedure. It is the aim of the journal to provide a peer-reviewed forum for the publication of original papers and authoritative review and opinion papers dealing with the most important issues facing the use of biomaterials in clinical practice. The scope of the journal covers the wide range of physical, biological and chemical sciences that underpin the design of biomaterials and the clinical disciplines in which they are used. These sciences include polymer synthesis and characterization, drug and gene vector design, the biology of the host response, immunology and toxicology and self assembly at the nanoscale. Clinical applications include the therapies of medical technology and regenerative medicine in all clinical disciplines, and diagnostic systems that reply on innovative contrast and sensing agents. The journal is relevant to areas such as cancer diagnosis and therapy, implantable devices, drug delivery systems, gene vectors, bionanotechnology and tissue engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


