Adipocyte Angptl8 deletion improves glucose and energy metabolism and obesity associated inflammation in mice
IF 4.6
2区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Angiopoietin-like protein 8 (Angptl8), expressed in the liver and adipocytes, forms a complex with Angptl3 or Angptl4, which regulates lipoprotein lipase and triglyceride metabolism. However, the precise functions of adipocyte Angptl8 remain elusive. Here we report that adipocyte-specific inducible Angptl8-knockout (AT-A8-KO) male mice on normal diet showed minor phenotypic changes, but after a high-fat high fructose (HFHF) diet, exhibited decreased body weight gain and glycemia, elevated rectal temperature and early dark phase energy expenditure compared to the Cre controls. AT-A8-KO mice also displayed improved glucose tolerance, a trend for better insulin sensitivity, improved insulin-stimulated glucose uptake in adipose tissues, and reduced visceral adipose tissue crown-like structures, plasma MCP-1 and leptin levels. The results indicate the importance of adipose Angptl8 in the context of nutri-stress and obesity, as its deletion in mice promotes a metabolically healthy obese phenotype by slightly ameliorating obesity, improving glucose and energy homeostasis, and mitigating inflammation.
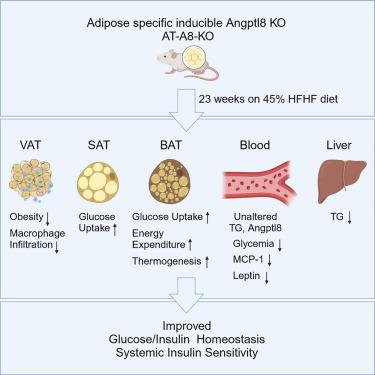
脂肪细胞 Angptl8 基因缺失可改善小鼠的葡萄糖和能量代谢以及与肥胖相关的炎症反应
血管生成素样蛋白8(Angptl8)在肝脏和脂肪细胞中表达,与Angptl3或Angptl4形成复合物,调节脂蛋白脂肪酶和甘油三酯的代谢。然而,脂肪细胞中 Angptl8 的确切功能仍然难以捉摸。在这里,我们报告了脂肪细胞特异性诱导性 Angptl8 基因敲除(AT-A8-KO)雄性小鼠在正常饮食中表现出轻微的表型变化,但在高脂高果糖(HFHF)饮食后,与 Cre 对照组相比,表现出体重增加和血糖下降、直肠温度升高和早期暗相能量消耗增加。AT-A8-KO 小鼠的葡萄糖耐量也有所改善,胰岛素敏感性有提高的趋势,胰岛素刺激脂肪组织摄取葡萄糖的情况有所改善,内脏脂肪组织冠状结构、血浆 MCP-1 和瘦素水平有所降低。这些结果表明了脂肪组织 Angptl8 在营养应激和肥胖方面的重要性,因为在小鼠体内删除 Angptl8 可轻微改善肥胖、改善葡萄糖和能量平衡并减轻炎症,从而促进代谢健康的肥胖表型。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

iScience
Multidisciplinary-Multidisciplinary
CiteScore
7.20
自引率
1.70%
发文量
1972
审稿时长
6 weeks
期刊介绍:
Science has many big remaining questions. To address them, we will need to work collaboratively and across disciplines. The goal of iScience is to help fuel that type of interdisciplinary thinking. iScience is a new open-access journal from Cell Press that provides a platform for original research in the life, physical, and earth sciences. The primary criterion for publication in iScience is a significant contribution to a relevant field combined with robust results and underlying methodology. The advances appearing in iScience include both fundamental and applied investigations across this interdisciplinary range of topic areas. To support transparency in scientific investigation, we are happy to consider replication studies and papers that describe negative results.
We know you want your work to be published quickly and to be widely visible within your community and beyond. With the strong international reputation of Cell Press behind it, publication in iScience will help your work garner the attention and recognition it merits. Like all Cell Press journals, iScience prioritizes rapid publication. Our editorial team pays special attention to high-quality author service and to efficient, clear-cut decisions based on the information available within the manuscript. iScience taps into the expertise across Cell Press journals and selected partners to inform our editorial decisions and help publish your science in a timely and seamless way.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


