Amorphous titanium dioxide with synergistic effect of nitrogen doping and oxygen vacancies by photoexcited sol-gel preparation for enhanced photodegradation of tetracycline
IF 4.1
3区 化学
Q2 CHEMISTRY, PHYSICAL
Journal of Photochemistry and Photobiology A-chemistry
Pub Date : 2024-11-20
DOI:10.1016/j.jphotochem.2024.116165
引用次数: 0
Abstract
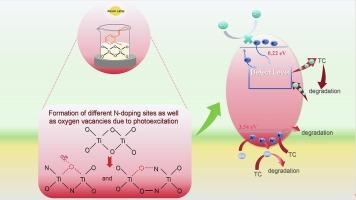
In this paper, a novel N-doped amorphous titanium dioxide (N20AT-hν) photocatalyst was prepared by the sol–gel route in which the sol added with salicylaldehyde hydrazone undergoes gel after photoexcitation. Based on a systematic exploration of as-prepared catalysts, it is known that N20AT-hν sample has a particle size of 50–60 nm, a specific surface area of 294.699 m2 g−1, and which is doped with nitrogen elements and forms abundant oxygen vacancies (OVs). The photocatalytic degradation rate of N20AT-hν for 20 mg L−1 tetracycline solution reached 92.23 % after 60 min, and the rate exhibited a slight decrease of 8.68 % after five cycles. Reactive species trapping experiments and electron spin resonance techniques indicate that superoxide radicals and holes are instrumental in photocatalytic degradation. Possible mechanism of forming the active species through OVs mediated traps and electron transfer pathways has been proposed. Furthermore, the predictive evaluations suggest that the degradation of tetracycline solutions by N20AT-hν sample results in less harmful decomposition product. Benefiting from the environmental friendliness of preparation method and the superiority of elemental doping, N20AT-hν material with synergistic effects has great potential for environmental applications.
通过光激发溶胶-凝胶法制备具有氮掺杂和氧空位协同效应的无定形二氧化钛,增强四环素的光降解能力
本文采用溶胶-凝胶法制备了一种新型 N 掺杂无定形二氧化钛(N20AT-hν)光催化剂。通过对制备的催化剂进行系统研究,可以得知 N20AT-hν 样品的粒径为 50-60 nm,比表面积为 294.699 m2 g-1,并且掺杂了氮元素,形成了丰富的氧空位(OVs)。60 分钟后,N20AT-hν 对 20 mg L-1 四环素溶液的光催化降解率达到 92.23%,五个循环后降解率略有下降,为 8.68%。反应物捕获实验和电子自旋共振技术表明,超氧自由基和空穴在光催化降解过程中起着重要作用。提出了通过 OVs 介导的陷阱和电子转移途径形成活性物种的可能机制。此外,预测性评估表明,N20AT-hν 样品对四环素溶液的降解会产生较少的有害分解产物。得益于制备方法的环境友好性和元素掺杂的优越性,具有协同效应的 N20AT-hν 材料在环境应用方面具有巨大潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.90
自引率
7.00%
发文量
580
审稿时长
48 days
期刊介绍:
JPPA publishes the results of fundamental studies on all aspects of chemical phenomena induced by interactions between light and molecules/matter of all kinds.
All systems capable of being described at the molecular or integrated multimolecular level are appropriate for the journal. This includes all molecular chemical species as well as biomolecular, supramolecular, polymer and other macromolecular systems, as well as solid state photochemistry. In addition, the journal publishes studies of semiconductor and other photoactive organic and inorganic materials, photocatalysis (organic, inorganic, supramolecular and superconductor).
The scope includes condensed and gas phase photochemistry, as well as synchrotron radiation chemistry. A broad range of processes and techniques in photochemistry are covered such as light induced energy, electron and proton transfer; nonlinear photochemical behavior; mechanistic investigation of photochemical reactions and identification of the products of photochemical reactions; quantum yield determinations and measurements of rate constants for primary and secondary photochemical processes; steady-state and time-resolved emission, ultrafast spectroscopic methods, single molecule spectroscopy, time resolved X-ray diffraction, luminescence microscopy, and scattering spectroscopy applied to photochemistry. Papers in emerging and applied areas such as luminescent sensors, electroluminescence, solar energy conversion, atmospheric photochemistry, environmental remediation, and related photocatalytic chemistry are also welcome.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


