Lysophosphatidylcholine induced by fat transplantation regulates hyperalgesia by affecting the dysfunction of ACC perineuronal nets
IF 4.6
2区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
The pathogenesis of hyperalgesia is complex and can lead to poor clinical treatment. Our study revealed that epididymal white adipose tissue (eWAT) from spared nerve injury (SNI) mice is involved in the occurrence of hyperalgesia after adipose tissue transplantation. We also showed that lysophosphatidylcholine (LPC) is enriched in the eWAT of SNI mice using non-targeted metabolomic analysis and verified that the levels of LPC in plasma and the anterior cingulate cortex (ACC) region increased following eWAT transplantation. Based on the immunohistochemistry results, we observed that LPC in the ACC region activated microglia via the TRPV1/CamkⅡ pathway. Meanwhile, the disruption of perineuronal nets (PNNs) around PV+ neurons in ACC promoted hyperalgesia, and the loss of PNNs and PV+ interneurons might be due to microglial phagocytosis. These findings elucidate the mechanism underlying hyperalgesia from the perspective of lipid metabolite LPC and PNNs and provide potential strategies for the treatment of hyperalgesia.
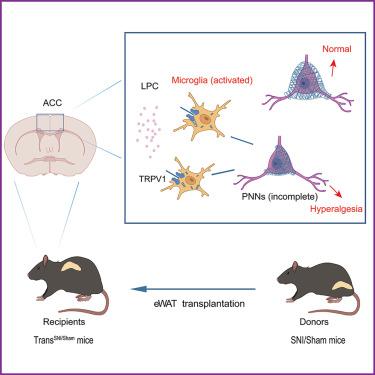
脂肪移植诱导的溶血磷脂酰胆碱通过影响 ACC 神经元周围网的功能障碍来调节痛觉减退
痛觉减退的发病机制十分复杂,可能导致临床治疗效果不佳。我们的研究发现,幸免神经损伤(SNI)小鼠的附睾白色脂肪组织(eWAT)参与了脂肪组织移植后过痛的发生。我们还利用非靶向代谢组学分析表明,溶血磷脂酰胆碱(LPC)富集于SNI小鼠的eWAT中,并验证了eWAT移植后血浆和前扣带皮层(ACC)区域的LPC水平升高。根据免疫组化结果,我们观察到 ACC 区域的 LPC 通过 TRPV1/CamkⅡ 通路激活了小胶质细胞。同时,ACC中PV+神经元周围神经元网络(PNNs)的破坏促进了痛觉减退,而PNNs和PV+中间神经元的丢失可能是由于小胶质细胞的吞噬作用造成的。这些发现从脂质代谢物LPC和PNNs的角度阐明了痛觉减退的机制,并为治疗痛觉减退提供了潜在的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

iScience
Multidisciplinary-Multidisciplinary
CiteScore
7.20
自引率
1.70%
发文量
1972
审稿时长
6 weeks
期刊介绍:
Science has many big remaining questions. To address them, we will need to work collaboratively and across disciplines. The goal of iScience is to help fuel that type of interdisciplinary thinking. iScience is a new open-access journal from Cell Press that provides a platform for original research in the life, physical, and earth sciences. The primary criterion for publication in iScience is a significant contribution to a relevant field combined with robust results and underlying methodology. The advances appearing in iScience include both fundamental and applied investigations across this interdisciplinary range of topic areas. To support transparency in scientific investigation, we are happy to consider replication studies and papers that describe negative results.
We know you want your work to be published quickly and to be widely visible within your community and beyond. With the strong international reputation of Cell Press behind it, publication in iScience will help your work garner the attention and recognition it merits. Like all Cell Press journals, iScience prioritizes rapid publication. Our editorial team pays special attention to high-quality author service and to efficient, clear-cut decisions based on the information available within the manuscript. iScience taps into the expertise across Cell Press journals and selected partners to inform our editorial decisions and help publish your science in a timely and seamless way.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


