Chitosan-based multimodal polymeric nanoparticles targeting pancreatic β-cells
IF 6.2
Q1 CHEMISTRY, APPLIED
Carbohydrate Polymer Technologies and Applications
Pub Date : 2024-11-19
DOI:10.1016/j.carpta.2024.100610
引用次数: 0
Abstract
Multimodal in vivo imaging of pancreatic islets might improve monitoring of endocrine grafts upon implantation, helping clinical validation of new regenerative therapies based on the replacement of β-cells in type 1 diabetes affected patients. Herein, the generation of chitosan-based multimodal diagnostic nanoparticles (NPs) able to target β-cells is described. The NPs, composed of chitosan (CH) and γ-poly-glutamic-acid (γ-PGA) with different “clickable” functional groups were chemoselectively decorated at the surface with Exendin-4 (Ex4), a ligand of glucagon-like peptide 1 (GLP-1) β-cell receptors, and with a DOTA containing linker, to chelate diagnostic radioisotopes. Furthermore, the NPs were conjugated with IRDye®800CW for multispectral optoacoustic tomography (MSOT). The affinity of Ex4 decorated NPs towards GLP-1R was confirmed by competitive flow cytometry tests. The detectability of the NPs labeled with IRDye®800CW and Ex4 in MSOT experiments was demonstrated. In vivo biodistribution of Ex4 decorated NPs labelled with Ga-68 was studied with positron emission tomography (PET) experiments in mice. Specific binding to GLP-1 receptor expressing tissue was demonstrated in autoradiography experiments, showing potential of the multimodal NPs for specifically targeting β-cells.
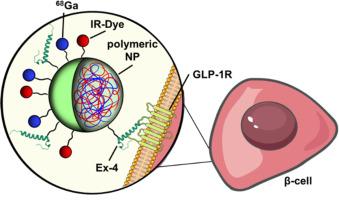
靶向胰腺β细胞的壳聚糖基多模式聚合物纳米粒子
胰岛的活体多模态成像可改善对内分泌移植物植入后的监测,有助于临床验证基于替代1型糖尿病患者β细胞的新型再生疗法。本文介绍了能靶向β细胞的壳聚糖基多模式诊断纳米粒子(NPs)的生成过程。这种由壳聚糖(CH)和γ-聚谷氨酸(γ-PGA)组成的 NPs 具有不同的 "可点击 "功能基团,其表面用胰高血糖素样肽 1(GLP-1)β 细胞受体配体 Exendin-4 (Ex4) 和含有 DOTA 的连接体进行化学选择性装饰,以螯合诊断放射性同位素。此外,NPs 还与 IRDye®800CW 共轭,用于多谱段光声断层扫描(MSOT)。竞争性流式细胞仪测试证实了经 Ex4 修饰的 NPs 对 GLP-1R 的亲和力。用 IRDye®800CW 和 Ex4 标记的 NPs 在 MSOT 实验中的可检测性也得到了证实。正电子发射断层扫描(PET)实验研究了用 Ga-68 标记的 Ex4 装饰 NPs 在小鼠体内的生物分布情况。自显影实验证明了与 GLP-1 受体表达组织的特异性结合,显示了多模式 NPs 特异性靶向 β 细胞的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


