One step derivatization and switchable hydrophilicity solvent-based microextraction for the determination of adamantane analogues in human urine by HPLC-FLD
IF 5.2
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
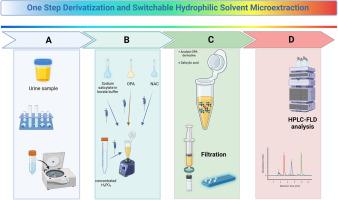
The present study describes an “one-step” derivatization and microextraction using a pH-switchable hydrophilicity solvent for the determination of amantadine and memantine in human urine by liquid chromatography and fluorescence detection. The procedure is based on the derivatization of the analytes with o-phthalaldehyde/N-acetyl cysteine at alkaline conditions in the presence of sodium salicylate as extractant in a homogeneous solution. The liquid-solid transition of salicylic acid was achieved by adding an aliquot of concentrated phosphoric acid that enables efficient dispersion and phase separation in a single step. Due to the moderate melting point of salicylic acid, its solidification is carried out at room temperature without the need for sample cooling. Critical parameters affecting the efficiency of the derivatization reaction and the microextraction performance were investigated and optimized. The fluorescent analyte derivatives were monitored at λex/λem = 340/450 nm. The proposed method was validated in terms of specificity, linearity, precision and trueness. The method was linear in the range of 50–2000 ng mL−1 while the intraday and between days precision was less than 13.7% in all cases. The trueness of the method ranged between 87.9 and 113%. The green character and the applicability of the method were assessed using ComplexMoGAPI and BAGI tools. The developed analytical scheme presented satisfactory performance, and it could be applied in the analysis of selected drugs in human urine samples.
通过 HPLC-FLD 一步衍生化和可切换亲水性溶剂微萃取法测定人体尿液中的金刚烷类似物
本研究介绍了一种 "一步法 "衍生化和微萃取方法,使用一种 pH 值可调的亲水性溶剂,通过液相色谱法和荧光检测法测定人体尿液中的金刚烷胺和美金刚。该方法是在碱性条件下,以水杨酸钠为萃取剂,在均匀溶液中用邻苯二甲醛/N-乙酰半胱氨酸对被分析物进行衍生。水杨酸的液固转换是通过加入等量的浓磷酸实现的,这样就能在一个步骤中实现高效的分散和相分离。由于水杨酸的熔点适中,其凝固过程可在室温下进行,无需冷却样品。对影响衍生反应效率和微萃取性能的关键参数进行了研究和优化。在 λex/λem = 340/450 纳米波长下监测荧光分析衍生物。所提议的方法在特异性、线性、精密度和真实性方面都得到了验证。该方法在 50-2000 ng mL-1 范围内线性良好,日内和日间精密度均小于 13.7%。该方法的准确度在 87.9%和 113%之间。使用ComplexMoGAPI和BAGI工具对该方法的绿色特性和适用性进行了评估。所开发的分析方案性能令人满意,可用于人体尿样中特定药物的分析。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


