In silico analysis of point mutation (c.687dupC; p. Met230Hisfs∗6) in PGAM2 gene that causes Glycogen Storage Disease (GSD) Type X
IF 1.2
4区 综合性期刊
Q3 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
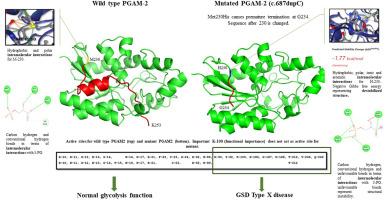
Glycogen storage disease (GSD) type X is an autosomal recessive disorder that affects skeletal muscles and is caused by PGAM-2 (Phosphoglycerate Mutase-2) enzyme deficiency. This deficiency is due to a mutation in the PGAM-2 gene at chromosome number 7p13. A novel insertion mutation (c.687dupC) was reported recently in the Pakistani population. This study aimed to computationally study the effect of that mutation at the molecular level. Several in silico approaches were employed to understand the molecular mechanism behind PGAM2 enzyme deficiency. Modeling the wild-type and mutant PGAM2 protein revealed an absence of an alpha helix from the c-terminus. Binding site analysis showed the absence of a critical residue, Lysine-100, from the mutant. Moreover, changes in the binding affinities, intramolecular interactions, and intermolecular interactions were also observed.
导致糖原贮积症(GSD)X 型的 PGAM2 基因点突变(c.687dupC; p. Met230Hisfs∗6)的硅学分析
糖原贮积病(GSD)X 型是一种常染色体隐性遗传疾病,会影响骨骼肌,是由 PGAM-2(磷酸甘油酸突变酶-2)酶缺乏症引起的。这种缺乏症是由于染色体 7p13 上的 PGAM-2 基因发生突变所致。最近在巴基斯坦人群中发现了一种新的插入突变(c.687dupC)。本研究旨在通过计算研究该突变在分子水平上的影响。为了了解 PGAM2 酶缺乏症背后的分子机制,我们采用了几种硅学方法。对野生型和突变型 PGAM2 蛋白进行建模后发现,其 c 端缺少一个 alpha 螺旋。结合位点分析表明,突变体缺少一个关键残基赖氨酸-100。此外,还观察到了结合亲和力、分子内相互作用和分子间相互作用的变化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Kuwait Journal of Science
MULTIDISCIPLINARY SCIENCES-
CiteScore
1.60
自引率
28.60%
发文量
132
期刊介绍:
Kuwait Journal of Science (KJS) is indexed and abstracted by major publishing houses such as Chemical Abstract, Science Citation Index, Current contents, Mathematics Abstract, Micribiological Abstracts etc. KJS publishes peer-review articles in various fields of Science including Mathematics, Computer Science, Physics, Statistics, Biology, Chemistry and Earth & Environmental Sciences. In addition, it also aims to bring the results of scientific research carried out under a variety of intellectual traditions and organizations to the attention of specialized scholarly readership. As such, the publisher expects the submission of original manuscripts which contain analysis and solutions about important theoretical, empirical and normative issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


