Insight into NH3-SCR and H2O/SO2 resistance of Ce modified iron-based catalysts with the presence of arsenic
IF 6.7
1区 工程技术
Q2 ENERGY & FUELS
引用次数: 0
Abstract
The mechanism of denitrification and arsenic poisoning during the selective catalytic reduction of NOx with ammonia using a cerium-modified iron-based catalyst was investigated through fixed bed activity tests and catalyst characterization. The results demonstrated that cerium formed a solid solution with Fe, and enriched the surface acid sites of the catalyst. Furthermore, cerium inhibited the arsenic-iron interaction, thereby protecting the active sites of the catalyst. After 6 h of arsenic poisoning, the NOx conversion of 0.1Ce catalyst decreased by only about 30 % of that of Fe2O3 catalyst. The cerium-modified iron-based catalyst exhibited significantly better H2O resistance than the Fe2O3 catalyst at 300 °C, and increased as the temperature rose·H2O adsorbed onto the Lewis acid sites on the catalyst surface, converting them into Brønsted acid sites, which promoted arsenic adsorption and oxidation on the surface, thereby greatly reducing adsorption activity of the catalyst. In the presence of SO2 and As2O3 in the flue gas, the denitrification efficiency of the catalyst further decreased, though the reduction is less pronounced than in the presence of H2O and As2O3 alone, and shows a negative correlation with SO2 concentration.
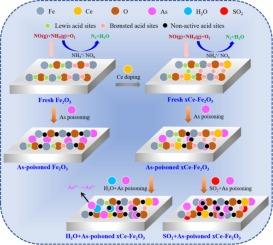
对含砷的 Ce 改性铁基催化剂耐 NH3-SCR 和 H2O/SO2 性能的深入研究
通过固定床活性试验和催化剂表征,研究了使用铈改性铁基催化剂选择性催化还原氨氧化氮过程中脱硝和砷中毒的机理。结果表明,铈与铁形成固溶体,丰富了催化剂的表面酸性位点。此外,铈还抑制了砷与铁的相互作用,从而保护了催化剂的活性位点。砷中毒 6 小时后,0.1Ce 催化剂的氮氧化物转化率仅为 Fe2O3 催化剂的 30%。铈改性铁基催化剂在 300 ℃ 时的抗 H2O 能力明显优于 Fe2O3 催化剂,并且随着温度的升高,抗 H2O 能力增强--H2O 吸附在催化剂表面的路易斯酸位点上,使其转化为布氏酸位点,从而促进了砷在催化剂表面的吸附和氧化,从而大大降低了催化剂的吸附活性。在烟气中存在 SO2 和 As2O3 的情况下,催化剂的脱硝效率进一步降低,但降低程度不如单独存在 H2O 和 As2O3 时明显,且与 SO2 浓度呈负相关。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Fuel
工程技术-工程:化工
CiteScore
12.80
自引率
20.30%
发文量
3506
审稿时长
64 days
期刊介绍:
The exploration of energy sources remains a critical matter of study. For the past nine decades, fuel has consistently held the forefront in primary research efforts within the field of energy science. This area of investigation encompasses a wide range of subjects, with a particular emphasis on emerging concerns like environmental factors and pollution.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


