Enhancing H+ intercalation kinetics and stability in Cu2+ pre-intercalated δ-MnO2 for aqueous aluminum batteries
IF 13.1
1区 化学
Q1 Energy
引用次数: 0
Abstract
Aqueous aluminum ion batteries (AAIBs) have garnered extensive attention due to their environmental friendliness, high theoretical capacity, and low cost. However, the sluggish reaction kinetics and severe structural collapse of the cathode material, especially manganese oxide, during the cycling process have hindered its further application. Herein, Cu2+ pre-intercalated layered δ-MnO2 was synthesized via a hydrothermal method. The pre-intercalated Cu2+ ions not only improve the conductivity of MnO2 cathode but also stabilize the structure to enhance stability. X-ray absorption fine structure (XAFS) combined with density functional theory (DFT) calculations confirm the formation of the covalent bond between Cu and O, increasing the electronegativity of O atoms and enhancing the H+ adsorption energy. Moreover, ex-situ measurements not only elucidate the Al3+/H+ co-insertion energy storage mechanism but also demonstrate the high reversibility of the Cu-MnO2 cathode during cycling. This work provides a promising modification approach for the application of manganese oxides in AAIBs.
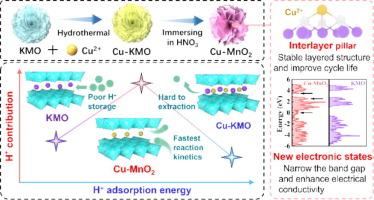
增强水性铝电池中 Cu2+ 预掺杂 δ-MnO2 的 H+ 插层动力学和稳定性
水性铝离子电池(AAIBs)因其环保、理论容量高和成本低而受到广泛关注。然而,在循环过程中,阴极材料(尤其是氧化锰)反应迟缓、结构严重坍塌,阻碍了其进一步应用。在此,我们通过水热法合成了 Cu2+ 预叠层 δ-MnO2。预镶层的 Cu2+ 离子不仅提高了 MnO2 阴极的导电性,而且稳定了结构,增强了稳定性。X 射线吸收精细结构(XAFS)结合密度泛函理论(DFT)计算证实了 Cu 和 O 之间形成共价键,从而提高了 O 原子的电负性,增强了 H+的吸附能。此外,原位测量不仅阐明了 Al3+/H+ 共插入储能机制,还证明了 Cu-MnO2 阴极在循环过程中的高可逆性。这项工作为锰氧化物在 AAIB 中的应用提供了一种前景广阔的改性方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


