Breath inspired multifunctional low-cost inorganic colloidal electrolyte for stable zinc metal anode
IF 13.1
1区 化学
Q1 Energy
引用次数: 0
Abstract
The practical application of aqueous zinc-ion batteries (AZIBs) is primarily constrained by issues such as corrosion, zinc dendrite formation, and the hydrogen evolution reaction occurring at the zinc metal anode. To overcome these challenges, strategies for optimizing the electrolyte are crucial for enhancing the stability of the zinc anode. Inspired by the role of hemoglobin in blood cells, which facilitates oxygen transport during human respiration, an innovative inorganic colloidal electrolyte has been developed: calcium silicate-ZnSO4 (denoted as CS-ZSO). This electrolyte operates in weak acidic environment and releases calcium ions, which participate in homotopic substitution with zinc ions, while the solvation environment of hydrated zinc ions in the electrolyte is regulated. The reduced energy barrier for the transfer of zinc ions and the energy barrier for the desolvation of hydrated ions imply faster ion transfer kinetics and accelerated desolvation processes, thus favoring the mass transfer process. Furthermore, the silicate colloidal particles act as lubricants, improving the transfer of zinc ions. Together, these factors contribute to the more uniform concentration of zinc ions at the electrode/electrolyte interface, effectively inhibiting zinc dendrite formation and reducing by-product accumulation. The Zn//CS-ZSO//Zn symmetric cell demonstrates stable operation for over 5000 h at 1 mA cm−2, representing 29-fold improvement compared to the Zn//ZSO//Zn symmetric cell, which lasts only 170 h. Additionally, the Zn//CS-ZSO//Cu asymmetric cell shows stable average Coulombic efficiency (CE) exceeding 99.6% over 2400 cycles, significantly surpassing the performance of the ZSO electrolyte. This modification strategy for electrolytes not only addresses key limitations associated with zinc anodes but also provides valuable insights into stabilizing anodes for the advancement of high-performance aqueous zinc-ion energy storage systems.
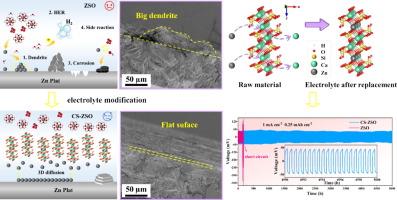
用于稳定锌金属阳极的呼吸启发式多功能低成本无机胶体电解质
锌离子水电池(AZIBs)的实际应用主要受到腐蚀、锌枝晶形成和锌金属阳极发生氢进化反应等问题的制约。为了克服这些挑战,优化电解质的策略对于提高锌阳极的稳定性至关重要。血红蛋白在血细胞中起着促进人类呼吸过程中氧气运输的作用,受此启发,一种创新的无机胶体电解质应运而生:硅酸钙-ZnSO4(简称 CS-ZSO)。这种电解质可在弱酸性环境中工作,并释放出钙离子,钙离子参与锌离子的同位取代,同时调节电解质中水合锌离子的溶解环境。锌离子转移的能量障碍和水合离子脱溶的能量障碍降低,意味着离子转移动力学加快,脱溶过程加速,从而有利于传质过程。此外,硅酸盐胶体颗粒还能起到润滑剂的作用,改善锌离子的转移。这些因素共同作用,使电极/电解质界面上的锌离子浓度更加均匀,有效抑制了锌枝晶的形成,减少了副产物的积累。Zn//CS-ZSO//Zn 对称电池在 1 mA cm-2 电流条件下可稳定工作 5000 小时以上,与仅能工作 170 小时的 Zn//ZSO//Zn 对称电池相比,性能提高了 29 倍。这种电解质改性策略不仅解决了锌阳极的主要局限性,还为稳定阳极以促进高性能水性锌离子储能系统的发展提供了宝贵的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


