High-valence Co deposition based on selfcatalysis of lattice Mn doping for robust acid water oxidation
IF 13.1
1区 化学
Q1 Energy
引用次数: 0
Abstract
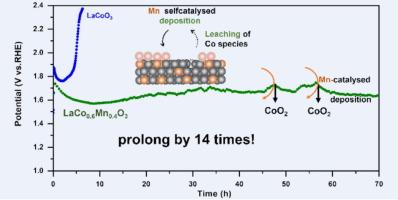
Non-precious metal cobalt-based oxide inevitably dissolves for acid oxygen evolution reaction (OER). Designing an efficient deposition channel for leaching cobalt species is a promising approach. The dissolution-deposition equilibrium of Co is achieved by doping Mn in the lattice of LaCo1−xMnxO3, prolonging the lifespan in acidic conditions by 14 times. The lattice doping of Mn produces a strain that enhances the adsorption capacity of OH−. The self-catalysis of Mn causes the leaching Co to be deposited in the form of CoO2, which ensures that the long-term stability of LaCo1−xMnxO3 is 70 h instead of 5 h for LaCoO3. Mn doping enhances the deprotonation of *OOH → O2 in acidic environments. Notably, the overpotential of optimized LaCo1−xMnxO3 is 345 mV at 10 mA cm−2 for acidic OER. This work presents a promising method for developing noble metal-free catalysts that enhance the acidic OER activity and stability.
基于晶格掺杂锰的自催化的高价钴沉积,用于强酸水氧化
非贵金属钴基氧化物在酸性氧进化反应(OER)中不可避免地会发生溶解。设计一种有效的沉积渠道来浸出钴是一种很有前景的方法。通过在 LaCo1-xMnxO3 晶格中掺杂锰,实现了钴的溶解-沉积平衡,从而将其在酸性条件下的寿命延长了 14 倍。锰的晶格掺杂产生的应变增强了对 OH- 的吸附能力。锰的自催化作用使浸出的钴以 CoO2 的形式沉积,这确保了 LaCo1-xMnxO3 的长期稳定性为 70 小时,而不是 LaCoO3 的 5 小时。在酸性环境中,锰的掺杂增强了 *OOH → O2 的去质子化作用。值得注意的是,经过优化的 LaCo1-xMnxO3 在 10 mA cm-2 的条件下,酸性 OER 的过电位为 345 mV。这项工作为开发可提高酸性 OER 活性和稳定性的无贵金属催化剂提供了一种可行的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


