pH modulation and molecular layer construction for stable zinc batteries
IF 13.1
1区 化学
Q1 Energy
引用次数: 0
Abstract
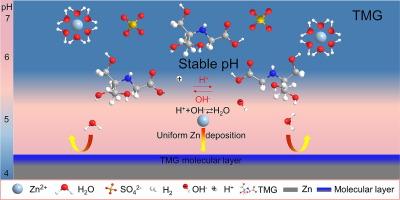
Aqueous zinc-ion batteries (AZIBs) have regained interest due to their inherent safety and cost-effectiveness. However, the zinc anode is notorious for side reactions and dendrite growth, which plague the practical application of AZIBs. Adjusting the interfacial pH to reduce the by-products has been proven to be effective in protecting the zinc anode. Nevertheless, the dynamic regulation of the inherently unstable zinc interface during prolonged cycling remains a significant challenge. Herein, zwitterionic N-tris(hydroxymethyl)methylglycine (TMG) integrated with negative –COO− and positive NH2+ groups is proposed to stabilize the Zn anode and extend the lifespan as a self-regulating interfacial additive. The anionic portion serves as a trapping site to balance the interfacial pH and thus mitigate the unintended side reactions. Simultaneously, the NH2+ cations are anchored on the zinc surface, forming a water-shielding, zincophilic molecular layer that guides three-dimensional diffusion and promotes uniform electro-deposition. Thus, an average plating efficiency of 99.74% over 3300 cycles at a current density of 2 mA cm−2 is achieved. Notably, the TMG additive actualizes ultralong life in Zn||Zn symmetrical cells (5500 h, exceeding 229 days, 1 mA cm−2/1 mA h cm−2), and enables the Zn||I2 cells to reach capacity retention rate of 89.4% after 1000 cycles at 1 A g−1.
用于稳定锌电池的 pH 值调节和分子层构造
锌离子水电池(AZIBs)因其固有的安全性和成本效益而重新受到关注。然而,锌阳极的副反应和枝晶生长是众所周知的,这困扰着 AZIB 的实际应用。事实证明,调节界面 pH 值以减少副产物可以有效保护锌阳极。然而,在长时间循环过程中如何动态调节本身就不稳定的锌界面仍然是一个重大挑战。在此,我们提出了含有负 -COO- 和正 NH2+ 基团的齐聚物 N-三(羟甲基)甲基甘氨酸 (TMG),作为一种自我调节界面添加剂,可稳定锌阳极并延长其使用寿命。阴离子部分可作为一个捕获点来平衡界面 pH 值,从而减轻意外的副反应。同时,NH2+ 阳离子锚定在锌表面,形成一个亲锌的水屏蔽分子层,引导三维扩散并促进均匀的电沉积。因此,在电流密度为 2 mA cm-2 的条件下,经过 3300 次循环,平均电镀效率达到 99.74%。值得注意的是,TMG 添加剂实现了 Zn||Zn 对称电池的超长寿命(5500 小时,超过 229 天,1 mA cm-2/1 mA h cm-2),并使 Zn||I2 电池在 1 A g-1 下循环 1000 次后容量保持率达到 89.4%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


