A potent moiety of (E)-5-(4-(5-chlorothiophene-2-carbonyl) piperazin-1-yl)-N'-(substituted methylene) benzofuran-2-carbohydrazide: Molecular docking, synthesis and antimicrobial evaluation
IF 3.4
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
In this paper, we report new hybrid molecules of (E)-5-(4-(5-chlorothiophene-2-carbonyl) piperazin-1-yl)-N'-(substituted methylene) benzofuran-2-carbohydrazide (10a-10r) as an anti-microbial agent. The synthesized compounds have been characterized by FT-IR, Mass, 1H and 13C NMR spectroscopic techniques and were evaluated on gram-positive (S. aureus and S. pyogenes), gram-negative (E. coli and P. aeruginosa) bacterial strain and fungal (C. albicans and A. Niger) strains by the broth dilution method. The compounds exhibited significant antibacterial and antifungal activities, out of which compounds 10b and 10o, having MIC 62.5 μg/mL, showed the best activity against gram-positive bacterial strain S. aureus. Furthermore, compounds 10e, 10j, and 10m, having MIC 62.5 μg/mL, showed the best activity against the gram-negative bacterial strain E. coli. In contrast, compounds 10g and 10n, having MIC 62.5 μg/mL, showed the best activity against gram-negative bacterial strain P. aeruginosa. Out of the series, compounds 10h and 10l have been found to possess better inhibitory activity with MIC 250 μg/mL, compared to standard drug Griseofulvin (MIC 500 μg/mL) against anti-fungal strain C. albicans. Additionally, the compounds were docked in DNA gyrase enzyme of gram-positive and gram-negative bacteria and also C. albicans fungal. Compounds 10h and 10l were docked with the CYP51 of C. albicans (PDB: 5V5Z), revealing that the benzyl ring of the carbohydrazide Schiff base formed a potent π-π stacking interaction with the Hem601 complex. Additionally, the carbonyl part of the amide functional group connecting the piperazine and thiophene rings was found to interact with His377 through a hydrogen bond, which is considered a significant interaction. The compounds are also checked for their drug-likeness property using Lipinski rule. On the basis of these findings, we can conclude that (E)-5-(4-(5-chlorothiophene-2-carbonyl) piperazin-1-yl)-N'-(substituted methylene) benzofuran-2-carbohydrazide is a potent antimicrobial agent against a variety of bacterial and fungal species.
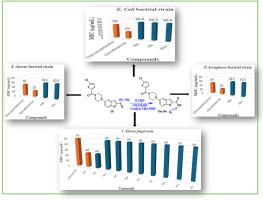
(E)-5-(4-(5-氯噻吩-2-羰基)哌嗪-1-基)-N'-(取代亚甲基)苯并呋喃-2-甲酰肼的强效分子:分子对接、合成和抗菌评价
本文报告了作为抗微生物剂的 (E)-5-(4-(5-氯噻吩-2-羰基)哌嗪-1-基)-N'-(取代亚甲基)苯并呋喃-2-甲酰肼(10a-10r)新杂交分子。合成的化合物通过傅立叶变换红外光谱、质谱、1H 和 13C NMR 光谱技术进行了表征,并通过肉汤稀释法对革兰氏阳性(金黄色葡萄球菌和化脓性葡萄球菌)、革兰氏阴性(大肠杆菌和绿脓杆菌)细菌菌株和真菌(白癣菌和尼日尔癣菌)菌株进行了评估。这些化合物具有明显的抗菌和抗真菌活性,其中化合物 10b 和 10o 的 MIC 值为 62.5 μg/mL,对革兰氏阳性细菌金黄色葡萄球菌的活性最好。此外,MIC 值为 62.5 μg/mL 的化合物 10e、10j 和 10m 对革兰氏阴性菌株大肠杆菌的活性最佳。相比之下,MIC 为 62.5 μg/mL 的化合物 10g 和 10n 对革兰氏阴性细菌铜绿假单胞菌的活性最佳。在这一系列化合物中,化合物 10h 和 10l 与标准药物 Griseofulvin(MIC 500 μg/mL)相比,对抗真菌菌株白僵菌具有更好的抑制活性,MIC 250 μg/mL。此外,这些化合物还与革兰氏阳性菌、革兰氏阴性菌和白僵菌的 DNA 回旋酶进行了对接。化合物 10h 和 10l 与白僵菌的 CYP51(PDB:5V5Z)进行了对接,发现羧酰肼席夫碱的苄基环与 Hem601 复合物形成了有效的 π-π 堆积相互作用。此外,还发现连接哌嗪环和噻吩环的酰胺官能团的羰基部分与 His377 通过氢键相互作用,这被认为是一种重要的相互作用。我们还利用利宾斯基规则检测了这些化合物的药物相似性。根据这些发现,我们可以得出结论:(E)-5-(4-(5-氯噻吩-2-羰基)哌嗪-1-基)-N'-(取代亚甲基)苯并呋喃-2-甲酰肼是一种针对多种细菌和真菌的强效抗菌剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


