Theoretical study on the performance of g-C3N4 loaded silver nanoparticles (Ag4, Ag8, Ag13) catalysts and their electrocatalytic reduction mechanism of CO2
IF 4.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
In this study, density functional theory (DFT) was employed to model Ag4, Ag8, and Ag13 nanoparticle-supported g-C3N4 catalysts. The stability of the Ag4, Ag8, and Ag13 nanoparticle-supported g-C3N4 catalysts was rigorously confirmed, followed by the computation of electron density and differential charge density profiles. The adsorption configuration of CO2 on the catalyst surfaces was optimized, revealing that the adsorption process is predominantly governed by chemisorption. It was determined that the activation of this process is intricately linked to the electronic interactions between CO2 molecules and the catalyst surface. Furthermore, the study explored the mechanisms underlying the electrochemical reduction of CO2, with a focus on elucidating the production pathways of four key products: HCOOH, CO, CH3OH, and CH4, across the three catalysts. The free energy profiles along the reaction pathways were analyzed to compare the selectivity and catalytic activity of the three catalysts for various reduction products. Among the catalysts studied, the Ag4-supported g-C3N4 catalyst exhibited the highest selectivity for the electrochemical reduction of CO2 to HCOOH. Additionally, the study demonstrated that an increase in the size of the Ag nanoparticles correlates with an enhanced selectivity for CO as a two-electron reduction product, while the catalytic activity for multi-electron reduction products such as HCOOH, CH3OH, and CH4 decreases. Band structure and density of states calculations for the three Ag nanoparticle-supported catalysts revealed an inverse correlation between band gap values and catalytic activity, with smaller band gaps associated with higher catalytic activity. This work provides critical insights into the relationship between Ag nanoparticle size and CO2 electrocatalytic reduction activity, offering a theoretical foundation for the design of advanced CO2 reduction catalysts.
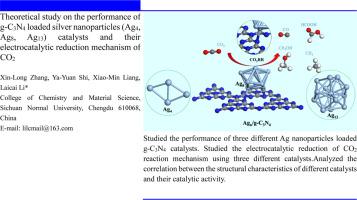
g-C3N4 负载银纳米粒子(Ag4、Ag8、Ag13)催化剂的性能及其对 CO2 的电催化还原机制的理论研究
本研究采用密度泛函理论(DFT)建立了 Ag4、Ag8 和 Ag13 纳米颗粒支撑的 g-C3N4 催化剂模型。通过计算电子密度和微分电荷密度曲线,严格确认了 Ag4、Ag8 和 Ag13 纳米颗粒支撑的 g-C3N4 催化剂的稳定性。对催化剂表面的二氧化碳吸附构型进行了优化,发现吸附过程主要由化学吸附作用控制。研究确定,这一过程的激活与二氧化碳分子和催化剂表面之间的电子相互作用密切相关。此外,该研究还探索了二氧化碳电化学还原的基本机制,重点阐明了四种关键产物的生成途径:HCOOH、CO、CH3OH 和 CH4。通过分析反应途径的自由能曲线,比较了三种催化剂对各种还原产物的选择性和催化活性。在所研究的催化剂中,Ag4 支持的 g-C3N4 催化剂在电化学还原 CO2 到 HCOOH 的过程中表现出最高的选择性。此外,研究还表明,Ag 纳米颗粒尺寸的增加与 CO 作为双电子还原产物的选择性增强相关,而 HCOOH、CH3OH 和 CH4 等多电子还原产物的催化活性则会降低。对三种银纳米粒子支撑催化剂的带结构和态密度计算表明,带隙值与催化活性之间存在反相关关系,带隙越小,催化活性越高。这项研究深入揭示了银纳米粒子尺寸与二氧化碳电催化还原活性之间的关系,为设计先进的二氧化碳还原催化剂奠定了理论基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


