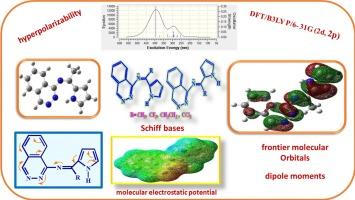
Molecular structures and optical properties of Schiff bases derived from pyrrole alkyl ketones and 1-aminophethalazine: DFT calculations
IF 2.5
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The density functional theory (DFT) method at the B3LYP/6-311++G (2d, 2p) level was used to compute the optimized structures of 1-aminophethalazine (I) derived Schiff bases of pyrrole alkyl ketones (II). In these Schiff bases (III, IV) each of the C![]() N bonds has two geometrical isomers (E and Z). The relative stability of these isomers has been calculated. Based on the optimized structures dipole moments, optical properties, UV–visible analysis, and frontier orbitals of these Schiff bases were computed. The values of electron density (ρ), Laplacian (∇2ρ), and potential energy density (V(r)) at the critical points (BCPs) of the carbon–nitrogen double bond (C
N bonds has two geometrical isomers (E and Z). The relative stability of these isomers has been calculated. Based on the optimized structures dipole moments, optical properties, UV–visible analysis, and frontier orbitals of these Schiff bases were computed. The values of electron density (ρ), Laplacian (∇2ρ), and potential energy density (V(r)) at the critical points (BCPs) of the carbon–nitrogen double bond (C![]() N) and intramolecular interaction were investigated. This is the first reported case of the molecular structures and optical characteristics of these Schiff bases using DFT calculations. The findings showed that these compounds had an almost identical magnitude of β value for p-nitroaniline, indicating good nonlinear optical characteristics.
N) and intramolecular interaction were investigated. This is the first reported case of the molecular structures and optical characteristics of these Schiff bases using DFT calculations. The findings showed that these compounds had an almost identical magnitude of β value for p-nitroaniline, indicating good nonlinear optical characteristics.
源自吡咯烷基酮和 1-aminophethalazine 的希夫碱的分子结构和光学特性:DFT 计算
在 B3LYP/6-311++G (2d, 2p) 水平上使用密度泛函理论(DFT)方法计算了 1-氨基酞嗪(I)衍生的吡咯烷基酮席夫碱(II)的优化结构。在这些希夫碱(III、IV)中,每个 CN 键都有两种几何异构体(E 和 Z)。对这些异构体的相对稳定性进行了计算。在优化结构的基础上,计算了这些席夫碱的偶极矩、光学性质、紫外可见光分析和前沿轨道。研究了碳氮双键(CN)临界点(BCPs)的电子密度(ρ)、拉普拉斯(∇2ρ)和势能密度(V(r))值以及分子内相互作用。这是首次报道利用 DFT 计算研究这些席夫碱的分子结构和光学特性。研究结果表明,这些化合物与对硝基苯胺的 β 值大小几乎相同,表明它们具有良好的非线性光学特性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


