Design, Synthesis, Characterization and antibacterial activity of new 1,2,3-triazole linked nucleosides
IF 2.5
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
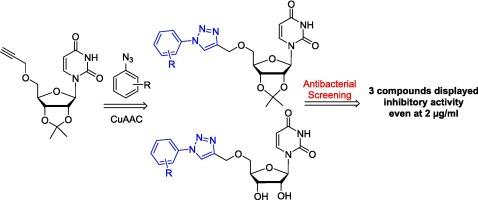
Sixteen new 1-aryl-1,2,3-triazole linked nucleosides (7a-7i, 8a-8 g) were designed and synthesized by using Copper (I)-catalysed Huisgen-Sharpless-Meldal 1,3-dipolar cycloaddition reaction of propargylated nucleoside with substituted fluoro aryl azides in good yields. All compounds (7a-7i, 8a-8 g) were characterized by analysis their 1H NMR, 13C NMR, 2D-NMR and mass spectral data. Compounds were screened in vitro for their antibacterial activity against Xanthomonas citri pv. malvacearum, Ralstonia solanacearum, Bacillus siamensis and Pseudomonas aeruginosa. All the synthesized compounds exhibited antibacterial potential against four bacterial strains in the range of 0.318 mm to 3.596 mm. On the basis of zone of inhibition, potential of compounds against bacteria was assessed by determining the MIC value of three compounds. The results indicated that three compounds 7f, 7 g and 8f were found effective even at minimal concentration of 2 µg/ml. Therefore development of such type of new antibacterial agents is the need of time in order to overcome the problem of resistance and modification may lead to the development of new antibacterial agents.
新型 1,2,3-三唑连接核苷的设计、合成、表征和抗菌活性
通过铜 (I) 催化的 Huisgen-Sharpless-Meldal 1,3-dipolar cycloaddition 反应,设计并合成了十六种新的 1-芳基-1,2,3-三唑连接核苷(7a-7i, 8a-8 g),丙炔化核苷与取代的氟芳基叠氮化物发生了良好的产率。通过分析 1H NMR、13C NMR、2D-NMR 和质谱数据,对所有化合物(7a-7i、8a-8 g)进行了表征。化合物对柠檬黄单胞菌(Xanthomonas citri pv.malvacearum)、茄黄杆菌(Ralstonia solanacearum)、暹罗芽孢杆菌(Bacillus siamensis)和铜绿假单胞菌(Pseudomonas aeruginosa)的抗菌活性进行了体外筛选。所有合成化合物对四种细菌菌株的抗菌潜力都在 0.318 毫米到 3.596 毫米之间。根据抑菌区,通过确定三种化合物的 MIC 值来评估化合物的抑菌潜力。结果表明,三种化合物 7f、7g 和 8f 即使在 2 µg/ml 的最小浓度下也有效。因此,为了克服抗药性问题,开发此类新型抗菌剂刻不容缓。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


