Synthetic Cu(III) from copper plating wastewater for onsite decomplexation of Cu(II)- and Ni(II)-organic complexes
IF 9.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Herein, the Cu(III) synthesized from copper plating effluent was developed for the first time to evaluate the onsite degradation performance of heavy metal complexes in the wastewater, thus achieving the purpose of “treating waste with waste”. The results indicated that synthetic Cu(III) presented the excellent decomplexation performance for Cu(II)/Ni(II)-organic complexes. The removal efficiency of Cu(II)/Ni(II)-EDTA significantly increased with increasing Cu(III) dosage, and the degradation of Cu(II)/Ni(II)-EDTA by synthetic Cu(III) system displayed highly pH-dependent reactivity. The radical quencher experiments confirmed that Cu(III) direct oxidation were mainly involved in the degradation of Cu(II)-EDTA. Additionally, the continuous decarboxylation process was proven to be the main degradation pathway of Cu(II)-EDTA in Cu(III) system. The coexisting substances (SO42−, Cl− and fulvic acids) showed little impacts at low level for the removal of Cu(II)/Ni(II)-EDTA, while retarded the degradation of Cu(II)-EDTA slightly at high level, which features high selective oxidation. Encouragingly, it was also effective to remove Cu(II)/Ni(II)-EDTA from in treating actual Cu/Ni-containing wastewater through synthetic Cu(III) treatment.
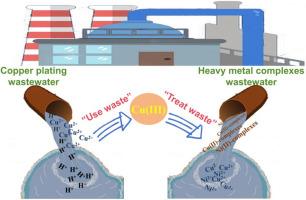
从镀铜废水中合成 Cu(III),用于现场解络合 Cu(II)-和 Ni(II)-有机络合物
本文首次开发了由镀铜废水合成的 Cu(III),用于评估废水中重金属络合物的现场降解性能,从而达到 "以废治废 "的目的。结果表明,合成的 Cu(III) 对 Cu(II)/Ni(II)- 有机络合物具有优异的解络合性能。随着 Cu(III) 用量的增加,Cu(II)/Ni(II)-EDTA 的去除率显著提高,而合成 Cu(III) 体系对 Cu(II)/Ni(II)-EDTA 的降解具有高度的 pH 依赖性。自由基淬灭剂实验证实,Cu(III) 直接氧化作用是 Cu(II)-EDTA 降解的主要原因。此外,连续脱羧过程被证明是 Cu(III)体系中 Cu(II)-EDTA 的主要降解途径。共存物质(SO42-、Cl- 和富勒酸)在低浓度时对 Cu(II)/Ni(II)-EDTA 的去除影响不大,而在高浓度时则略微延缓了 Cu(II)-EDTA 的降解,这说明其具有高选择性氧化的特点。令人鼓舞的是,在通过合成 Cu(III) 处理实际含铜/镍废水时,它也能有效去除 Cu(II)/Ni(II)-EDTA 中的 Cu(II)/Ni(II)-EDTA。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chinese Chemical Letters
化学-化学综合
CiteScore
14.10
自引率
15.40%
发文量
8969
审稿时长
1.6 months
期刊介绍:
Chinese Chemical Letters (CCL) (ISSN 1001-8417) was founded in July 1990. The journal publishes preliminary accounts in the whole field of chemistry, including inorganic chemistry, organic chemistry, analytical chemistry, physical chemistry, polymer chemistry, applied chemistry, etc.Chinese Chemical Letters does not accept articles previously published or scheduled to be published. To verify originality, your article may be checked by the originality detection service CrossCheck.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


