FeCl3⋅6H2O-mediated cyclization of fluorinated 2’-hydroxychalcones in alcohol medium into flavanones with antiviral activity
IF 1.7
4区 化学
Q3 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
A simple, inexpensive and efficient approach for the synthesis of fluorinated 2-arylchroman-4-ones from corresponding 2′-hydroxychalcones has been realized. The reactions are successfully conducted in presence of FeCl3⋅6H2O in EtOH at 100 °C. It was found that the same transformation takes place in the reaction system FeCl3⋅6H2O/MeOH/100 °C. In contrast, the use of FeCl3⋅6H2O in MeOH at 150 °C leads to the oxidative cyclization of 2′-hydroxychalcones to form 2-arylchromen-4-ones. A some of the obtained fluorinated flavanones were evaluated for their inhibitory activity against influenza A virus A/Puerto Rico/8/34 (H1N1) in the MDCK cell culture. Among the studied heterocycles 6,7-difluoro-2-(4-methoxyphenyl)chroman-4-one exhibited the highest anti-influenza virus activity (IC50 = 11.4 μM, SI = 91).
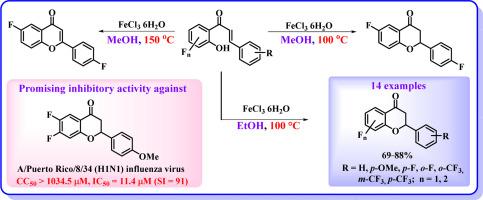
FeCl3⋅6H2O介导的氟化2'-羟基查耳酮在酒精介质中环化成具有抗病毒活性的黄烷酮类化合物
实现了从相应的 2′-羟基查耳酮合成氟化 2-芳基苯并吡喃-4-酮的简单、廉价和高效的方法。在 100 °C、EtOH 溶液中有 FeCl3⋅6H2O 的条件下,反应得以成功进行。研究发现,在 FeCl3⋅6H2O/MeOH/100 °C 反应体系中也发生了同样的转化。相反,在 150 °C、MeOH 中使用 FeCl3⋅6H2O 会导致 2′-羟基查耳酮氧化环化,生成 2-芳基苯并吡喃-4-酮。在 MDCK 细胞培养中,对获得的一些含氟黄酮对甲型流感病毒 A/Puerto Rico/8/34 (H1N1) 的抑制活性进行了评估。在所研究的杂环中,6,7-二氟-2-(4-甲氧基苯基)色满-4-酮的抗流感病毒活性最高(IC50 = 11.4 μM,SI = 91)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Fluorine Chemistry
化学-无机化学与核化学
CiteScore
3.80
自引率
10.50%
发文量
99
审稿时长
33 days
期刊介绍:
The Journal of Fluorine Chemistry contains reviews, original papers and short communications. The journal covers all aspects of pure and applied research on the chemistry as well as on the applications of fluorine, and of compounds or materials where fluorine exercises significant effects. This can include all chemistry research areas (inorganic, organic, organometallic, macromolecular and physical chemistry) but also includes papers on biological/biochemical related aspects of Fluorine chemistry as well as medicinal, agrochemical and pharmacological research. The Journal of Fluorine Chemistry also publishes environmental and industrial papers dealing with aspects of Fluorine chemistry on energy and material sciences. Preparative and physico-chemical investigations as well as theoretical, structural and mechanistic aspects are covered. The Journal, however, does not accept work of purely routine nature.
For reviews and special issues on particular topics of fluorine chemistry or from selected symposia, please contact the Regional Editors for further details.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


