Surface reconstruction of Co(OH)2 nanosheets through an in-situ PBA etching and sulfuration strategy for enhanced electrocatalytic oxygen evolution reaction
IF 3.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Designing an electrocatalyst for the oxygen evolution reaction (OER) that combines high activity with exceptional stability is a pivotal advancement in sustainable water splitting technology, heralding a novel approach to produce clean, high-purity hydrogen. This paper introduces an innovative strategy centered on the construction of a three-dimensional heterostructure supported on carbon cloth, specifically Fe–CoS2/Co(OH)2/CC. This meticulous strategy begins with the meticulous treatment of Co(OH)2 nanosheets, cleverly inducing the formation of Prussian blue-like compounds through in-situ etching techniques. Subsequently, a direct sulfuration treatment is employed, resulting in a delicate reconstruction of the Co(OH)2 nanosheet surface, ultimately yielding a unique Fe–CoS2/Co(OH)2/CC heterostructure. This composite material exhibits remarkable low overpotential performance, achieving an impressive 235 mV at a current density of 10 mA cm–2, which is comparable to that of established catalysts such as Ruthenium dioxide (RuO2). The exceptional catalytic activity of Fe–CoS2/Co(OH)2/CC is primarily attributed to its unique 3D structure, crafted through the in-situ growth of Prussian blue analogs (PBAs). This structure not only ensures uniform iron doping but also significantly enhances the material's stability and reactivity. Furthermore, the high conductivity of the self-supported carbon cloth electrode facilitates rapid electron transport, further augmenting the overall catalytic efficiency.
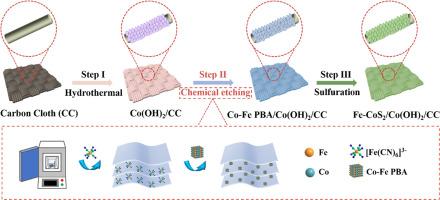
通过原位 PBA 刻蚀和硫化策略重构 Co(OH)2 纳米片表面,以增强电催化氧进化反应
设计一种兼具高活性和优异稳定性的氧进化反应(OER)电催化剂是可持续水分离技术的关键进步,它预示着一种生产清洁、高纯度氢气的新方法。本文介绍了一种创新战略,其核心是在碳布(特别是 Fe-CoS2/Co(OH)2/CC)上构建三维异质结构。这一缜密的策略首先是对 Co(OH)2 纳米片进行精细处理,通过原位蚀刻技术巧妙地诱导普鲁士蓝类化合物的形成。随后,采用直接硫化处理,对 Co(OH)2 纳米片表面进行精细的重构,最终形成独特的 Fe-CoS2/Co(OH)2/CC 异质结构。这种复合材料具有显著的低过电位性能,在 10 mA cm-2 的电流密度下可达到惊人的 235 mV,与二氧化钌(RuO2)等成熟催化剂的过电位性能相当。Fe-CoS2/Co(OH)2/CC 的卓越催化活性主要归功于其独特的三维结构,这种结构是通过普鲁士蓝类似物(PBA)的原位生长制作而成的。这种结构不仅确保了铁的均匀掺杂,还大大提高了材料的稳定性和反应活性。此外,自支撑碳布电极的高导电性还有利于电子的快速传输,进一步提高了整体催化效率。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


