Silver- and gold-catalyzed azide−alkyne cycloaddition by functionalized NHC-based polynuclear catalysts: Computational investigation and mechanistic insights
IF 3.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
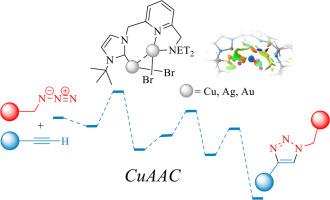
This study evaluated the catalytic efficiency of Au(I), Ag(I), and Cu(I) complexes in the azide‒alkyne cycloaddition (AAC) reaction through density functional theory (DFT) calculations. Cu(I) complexes exhibit superior catalytic performance, with lower energy barriers (8.8 kcal/mol) and a favorable Gibbs free energy of -0.9 kcal/mol in the key cycloaddition step, significantly outperforming Ag and Au complexes. Structural analysis revealed that shorter M−C bond lengths in the Cu complex contributed to increased stability. Additionally, the copper complex has a more negative Gibbs free energy for the formed metallacycle, indicating a thermodynamically favorable reaction pathway. Noncovalent interaction (NCI) and reduced density gradient (RDG) analyses of the Cu, Ag, and Au systems highlighted distinct interaction patterns influencing the reactivity. Furthermore, electron localization function (ELF) and localized orbital locator (LOL) analyses revealed bonding characteristics in those complexes. This study offers valuable insights into the mechanistic differences among Au(I), Ag(I), and Cu(I) complexes, paving the way for future research on enhancing the catalytic activity of copper, silver and gold complexes through ligand modification.
功能化 NHC 基多核催化剂催化的叠氮-炔环加成反应:计算研究和机理认识
本研究通过密度泛函理论(DFT)计算,评估了 Au(I)、Ag(I) 和 Cu(I) 复合物在叠氮-炔环加成反应(AAC)中的催化效率。Cu(I)配合物表现出卓越的催化性能,在关键的环加成反应步骤中,能垒较低(8.8 kcal/mol),吉布斯自由能为-0.9 kcal/mol,明显优于Ag和Au配合物。结构分析表明,铜配合物中较短的 M-C 键长度有助于提高稳定性。此外,铜配合物形成的金属环的吉布斯自由能为负值,这表明反应途径在热力学上是有利的。对铜、银和金体系进行的非共价相互作用(NCI)和还原密度梯度(RDG)分析凸显了影响反应活性的不同相互作用模式。此外,电子定位功能(ELF)和局部轨道定位器(LOL)分析揭示了这些复合物的成键特征。这项研究为了解 Au(I)、Ag(I) 和 Cu(I) 复合物之间的机理差异提供了宝贵的见解,为今后研究通过配体修饰提高铜、银和金复合物的催化活性铺平了道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


