Enantioconvergent copper-catalysed difluoromethylation of alkyl halides
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Stereochemically controlled hydrogen bond donors play essential roles in the pharmaceutical industry. Consequently, organic molecules that bear difluoromethyl (CF2H) groups at chiral centres are emerging as pivotal components in pharmaceuticals owing to their distinct hydrogen-bonding property. However, a general approach for introducing CF2H groups in an enantioselective manner has remained elusive. Here we show that enantioconvergent difluoromethylation of racemic alkyl electrophiles, through alkyl radical intermediates, represents a strategy for constructing CF2H-containing stereocentres. This strategy is enabled by using copper catalysts bound with a chiral diamine ligand bearing electron-deficient phenyl groups, and a nucleophilic CF2H-zinc reagent. This method allows the high-yield conversion of a diverse range of alkyl halides into their alkyl-CF2H analogues with excellent enantioselectivity. Mechanistic studies reveal a route involving asymmetric difluoromethylation of alkyl radicals and crucial non-covalent interactions in the enantiodetermining steps. This copper-catalysed difluoromethylation process opens an avenue for the efficient preparation of CF2H-containing pharmaceuticals. Despite the importance of difluoromethyl (CF2H)-bearing centres for pharmaceuticals, there is currently no general strategy for the stereoselective introduction of a CF2H group at chiral centres. Here the authors describe an enantioconvergent difluoromethylation method for racemic alkyl halides to construct such stereocentres.
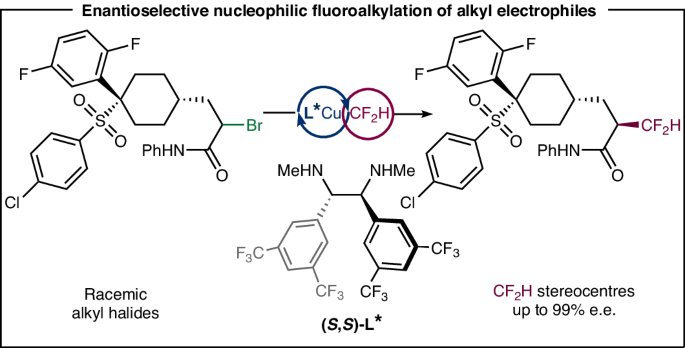

铜催化烷基卤的对映体二氟甲基化反应
立体化学控制的氢键供体在制药业中发挥着至关重要的作用。因此,在手性中心带有二氟甲基(CF2H)基团的有机分子因其独特的氢键特性而逐渐成为制药中的关键成分。然而,以对映选择性的方式引入 CF2H 基团的一般方法仍未出现。在这里,我们展示了一种通过烷基自由基中间体对消旋烷基亲电体进行对映转化的二氟甲基化反应,这是一种构建含 CF2H 立体中心的策略。使用铜催化剂与带有缺电子苯基的手性二胺配体和亲核 CF2H 锌试剂结合,可以实现这种策略。这种方法可以高产地将各种烷基卤化物转化为烷基-CF2H 类似物,并具有极佳的对映选择性。机理研究揭示了一条涉及烷基自由基不对称二氟甲基化和对映体决定步骤中关键的非共价相互作用的路线。这种铜催化的二氟甲基化过程为高效制备含 CF2H 的药物开辟了一条途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


