Self-Reinforced Cathode Interface to Prolong the Cyclic Stability of Zn-MnO2 Batteries
IF 9.1
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Rechargeable aqueous zinc-ion batteries (RAZIBs) are attracting increasing attention due to their advantages in safety, cost, and energy density. However, the choice of electrode binders when using aqueous electrolytes faces many constraints, as nontoxic and low-cost hydrophilic binders, such as sodium alginate (SA), can lead to electrode damage due to excessive swelling in aqueous solution. Here, by employing double-network hydrogel electrolytes formed by poly(vinyl alcohol) and alginate, the content and activity of water molecules at the electrode–electrolyte interface are reduced, and the mechanical stability of the electrode is reinforced, thereby affording reliable protection to the cathode bonded with SA. The quasi-solid-state interface formed between SA and the gel electrolyte provides stable electrode architecture and smooth ion transport, thus enabling Zn||MnO2 cells to exhibit excellent electrochemical cycling performance even when using hydrophilic binders.
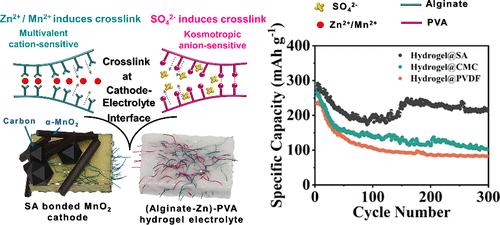
自我强化阴极界面以延长 Zn-MnO2 电池的循环稳定性
可充电锌离子水电池(RAZIBs)因其在安全性、成本和能量密度方面的优势而日益受到关注。然而,在使用水性电解质时,电极粘合剂的选择面临许多限制,因为无毒且低成本的亲水性粘合剂(如海藻酸钠(SA))会因在水溶液中过度膨胀而导致电极损坏。在这里,通过采用由聚(乙烯醇)和海藻酸盐形成的双网水凝胶电解质,降低了电极-电解质界面上水分子的含量和活性,增强了电极的机械稳定性,从而为与 SA 粘合的阴极提供了可靠的保护。SA 与凝胶电解质之间形成的准固态界面提供了稳定的电极结构和顺畅的离子传输,从而使 Zn||MnO2 电池即使在使用亲水性粘合剂的情况下也能表现出优异的电化学循环性能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nano Letters
工程技术-材料科学:综合
CiteScore
16.80
自引率
2.80%
发文量
1182
审稿时长
1.4 months
期刊介绍:
Nano Letters serves as a dynamic platform for promptly disseminating original results in fundamental, applied, and emerging research across all facets of nanoscience and nanotechnology. A pivotal criterion for inclusion within Nano Letters is the convergence of at least two different areas or disciplines, ensuring a rich interdisciplinary scope. The journal is dedicated to fostering exploration in diverse areas, including:
- Experimental and theoretical findings on physical, chemical, and biological phenomena at the nanoscale
- Synthesis, characterization, and processing of organic, inorganic, polymer, and hybrid nanomaterials through physical, chemical, and biological methodologies
- Modeling and simulation of synthetic, assembly, and interaction processes
- Realization of integrated nanostructures and nano-engineered devices exhibiting advanced performance
- Applications of nanoscale materials in living and environmental systems
Nano Letters is committed to advancing and showcasing groundbreaking research that intersects various domains, fostering innovation and collaboration in the ever-evolving field of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


